Safety and activity of anti-PD-L1 antibody in patients with advanced cancer
- PMID: 22658128
- PMCID: PMC3563263
- DOI: 10.1056/NEJMoa1200694
Safety and activity of anti-PD-L1 antibody in patients with advanced cancer
Abstract
Background: Programmed death 1 (PD-1) protein, a T-cell coinhibitory receptor, and one of its ligands, PD-L1, play a pivotal role in the ability of tumor cells to evade the host's immune system. Blockade of interactions between PD-1 and PD-L1 enhances immune function in vitro and mediates antitumor activity in preclinical models.
Methods: In this multicenter phase 1 trial, we administered intravenous anti-PD-L1 antibody (at escalating doses ranging from 0.3 to 10 mg per kilogram of body weight) to patients with selected advanced cancers. Anti-PD-L1 antibody was administered every 14 days in 6-week cycles for up to 16 cycles or until the patient had a complete response or confirmed disease progression.
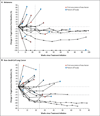
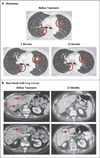
Results: As of February 24, 2012, a total of 207 patients--75 with non-small-cell lung cancer, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer--had received anti-PD-L1 antibody. The median duration of therapy was 12 weeks (range, 2 to 111). Grade 3 or 4 toxic effects that investigators considered to be related to treatment occurred in 9% of patients. Among patients with a response that could be evaluated, an objective response (a complete or partial response) was observed in 9 of 52 patients with melanoma, 2 of 17 with renal-cell cancer, 5 of 49 with non-small-cell lung cancer, and 1 of 17 with ovarian cancer. Responses lasted for 1 year or more in 8 of 16 patients with at least 1 year of follow-up.
Conclusions: Antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rates of 12 to 41% at 24 weeks) in patients with advanced cancers, including non-small-cell lung cancer, melanoma, and renal-cell cancer. (Funded by Bristol-Myers Squibb and others; ClinicalTrials.gov number, NCT00729664.).
Figures
Comment in
-
Tumor immunotherapy directed at PD-1.N Engl J Med. 2012 Jun 28;366(26):2517-9. doi: 10.1056/NEJMe1205943. Epub 2012 Jun 2. N Engl J Med. 2012. PMID: 22658126 No abstract available.
-
From ASCO-immunotherapy: programming cancer cell death.Nat Rev Clin Oncol. 2012 Jun 19;9(8):427. doi: 10.1038/nrclinonc.2012.104. Nat Rev Clin Oncol. 2012. PMID: 22710340 No abstract available.
-
The future of cancer treatment: will it include immunotherapy?Cancer Cell. 2012 Jul 10;22(1):7-8. doi: 10.1016/j.ccr.2012.06.009. Cancer Cell. 2012. PMID: 22789534
-
Re: Safety and activity of anti-PD-L1 antibody in patients with advanced cancer.J Urol. 2012 Dec;188(6):2148-9. doi: 10.1016/j.juro.2012.08.169. Epub 2012 Oct 18. J Urol. 2012. PMID: 23141220 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
