Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes
- PMID: 22659329
- PMCID: PMC3400836
- DOI: 10.1124/pr.111.005637
Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes
Abstract
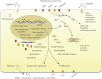
Cisplatin is one of the most effective broad-spectrum anticancer drugs. Its effectiveness seems to be due to the unique properties of cisplatin, which enters cells via multiple pathways and forms multiple different DNA-platinum adducts while initiating a cellular self-defense system by activating or silencing a variety of different genes, resulting in dramatic epigenetic and/or genetic alternations. As a result, the development of cisplatin resistance in human cancer cells in vivo and in vitro by necessity stems from bewilderingly complex genetic and epigenetic changes in gene expression and alterations in protein localization. Extensive published evidence has demonstrated that pleiotropic alterations are frequently detected during development of resistance to this toxic metal compound. Changes occur in almost every mechanism supporting cell survival, including cell growth-promoting pathways, apoptosis, developmental pathways, DNA damage repair, and endocytosis. In general, dozens of genes are affected in cisplatin-resistant cells, including pathways involved in copper metabolism as well as transcription pathways that alter the cytoskeleton, change cell surface presentation of proteins, and regulate epithelial-to-mesenchymal transition. Decreased accumulation is one of the most common features resulting in cisplatin resistance. This seems to be a consequence of numerous epigenetic and genetic changes leading to the loss of cell-surface binding sites and/or transporters for cisplatin, and decreased fluid phase endocytosis.
Figures

References
-
- Arts HJ, Hollema H, Lemstra W, Willemse PH, De Vries EG, Kampinga HH, Van der Zee AG. (1999) Heat-shock-protein-27 (hsp27) expression in ovarian carcinoma: relation in response to chemotherapy and prognosis. Int J Cancer 84:234–238 - PubMed
-
- Baum B, Settleman J, Quinlan MP. (2008) Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 19:294–308 - PubMed
-
- Belfi CA, Chatterjee S, Gosky DM, Berger SJ, Berger NA. (1999) Increased sensitivity of human colon cancer cells to DNA cross-linking agents after GRP78 up-regulation. Biochem Biophys Res Commun 257:361–368 - PubMed
-
- Beretta GL, Benedetti V, Cossa G, Assaraf YG, Bram E, Gatti L, Corna E, Carenini N, Colangelo D, Howell SB, et al. (2010) Increased levels and defective glycosylation of MRPs in ovarian carcinoma cells resistant to oxaliplatin. Biochem Pharmacol 79:1108–1117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

