The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome
- PMID: 22659346
- PMCID: PMC3667409
- DOI: 10.1016/j.jhep.2012.05.014
The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome
Abstract
Background & aims: Hepatopulmonary syndrome (HPS), classically attributed to intrapulmonary vascular dilatation, occurs in 15-30% of cirrhotics and causes hypoxemia and increases mortality. In experimental HPS after common bile duct ligation (CBDL), monocytes adhere in the lung vasculature and produce vascular endothelial growth factor (VEGF)-A and angiogenesis ensues and contribute to abnormal gas exchange. However, the mechanisms for these events are unknown. The chemokine fractalkine (CX(3)CL1) can directly mediate monocyte adhesion and activate VEGF-A and angiogenesis via its receptor CX(3)CR1 on monocytes and endothelium during inflammatory angiogenesis. We explored whether pulmonary CX(3)CL1/CX(3)CR1 alterations occur after CBDL and influence pulmonary angiogenesis and HPS.
Methods: Pulmonary CX(3)CL1/CX(3)CR1 expression and localization, CX(3)CL1 signaling pathway activation, monocyte accumulation, and development of angiogenesis and HPS were assessed in 2- and 4-week CBDL animals. The effects of a neutralizing antibody to CX(3)CR1 (anti-CX(3)CR1 Ab) on HPS after CBDL were evaluated.
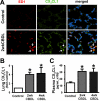
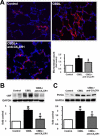
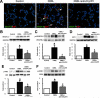
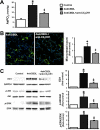
Results: Circulating CX(3)CL1 levels and lung expression of CX(3)CL1 and CX(3)CR1 in intravascular monocytes and microvascular endothelium increased in 2- and 4-week CBDL animals as HPS developed. These events were accompanied by pulmonary angiogenesis, monocyte accumulation, activation of CX(3)CL1 mediated signaling pathways (Akt, ERK) and increased VEGF-A expression and signaling. Anti-CX(3)CR1 Ab treatment reduced monocyte accumulation, decreased lung angiogenesis and improved HPS. These events were accompanied by inhibition of CX(3)CL1 signaling pathways and a reduction in VEGF-A expression and signaling.
Conclusions: Circulating CX(3)CL1 levels and pulmonary CX(3)CL1/CX(3)CR1 expression and signaling increase after CBDL and contribute to pulmonary intravascular monocyte accumulation, angiogenesis and development of experimental HPS.
Copyright © 2012 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
The role of receptor tyrosine kinase activation in cholangiocytes and pulmonary vascular endothelium in experimental hepatopulmonary syndrome.Am J Physiol Gastrointest Liver Physiol. 2014 Jan 1;306(1):G72-80. doi: 10.1152/ajpgi.00178.2013. Epub 2013 Nov 7. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24200956 Free PMC article.
-
Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome.Gastroenterology. 2009 Mar;136(3):1070-80. doi: 10.1053/j.gastro.2008.12.001. Epub 2008 Dec 3. Gastroenterology. 2009. PMID: 19109954 Free PMC article.
-
Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome.Am J Pathol. 2014 Jun;184(6):1706-14. doi: 10.1016/j.ajpath.2014.02.027. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24731444 Free PMC article.
-
CX3CL1/fractalkine is a novel regulator of normal and malignant human B cell function.J Leukoc Biol. 2012 Jul;92(1):51-8. doi: 10.1189/jlb.0112035. Epub 2012 Mar 27. J Leukoc Biol. 2012. PMID: 22457367 Review.
-
Hepatopulmonary syndrome: update on pathogenesis and clinical features.Nat Rev Gastroenterol Hepatol. 2012 Sep;9(9):539-49. doi: 10.1038/nrgastro.2012.123. Epub 2012 Jul 3. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22751459 Free PMC article. Review.
Cited by
-
Hepatopulmonary Syndrome and Liver Transplantation: A Recent Review of the Literature.J Clin Transl Hepatol. 2016 Mar 28;4(1):47-53. doi: 10.14218/JCTH.2015.00044. Epub 2016 Mar 15. J Clin Transl Hepatol. 2016. PMID: 27047772 Free PMC article. Review.
-
Pulmonary complications of hepatic diseases.World J Gastroenterol. 2016 Jul 14;22(26):6008-15. doi: 10.3748/wjg.v22.i26.6008. World J Gastroenterol. 2016. PMID: 27468192 Free PMC article. Review.
-
Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications.Nat Rev Cardiol. 2021 Feb;18(2):117-135. doi: 10.1038/s41569-020-0433-5. Epub 2020 Sep 30. Nat Rev Cardiol. 2021. PMID: 32999450 Review.
-
Endothelial glycocalyx in hepatopulmonary syndrome: An indispensable player mediating vascular changes.Front Immunol. 2022 Dec 22;13:1039618. doi: 10.3389/fimmu.2022.1039618. eCollection 2022. Front Immunol. 2022. PMID: 36618396 Free PMC article. Review.
-
Impact of hepatopulmonary syndrome in liver transplantation candidates and the role of angiogenesis.Eur Respir J. 2022 Aug 4;60(2):2102304. doi: 10.1183/13993003.02304-2021. Print 2022 Aug. Eur Respir J. 2022. PMID: 34949701 Free PMC article.
References
-
- Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome -- a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. - PubMed
-
- Schenk P, Schoniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. - PubMed
-
- Swanson K, Wiesner R, Krowka M. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122–1129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

