Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness
- PMID: 22659883
- PMCID: PMC3423859
- DOI: 10.1152/ajplung.00108.2012
Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness
Abstract
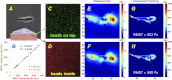
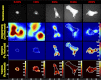
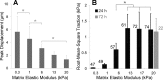
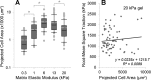
Lung fibroblast functions such as matrix remodeling and activation of latent transforming growth factor-β1 (TGF-β1) are associated with expression of the myofibroblast phenotype and are directly linked to fibroblast capacity to generate force and deform the extracellular matrix. However, the study of fibroblast force-generating capacities through methods such as traction force microscopy is hindered by low throughput and time-consuming procedures. In this study, we improved at the detail level methods for higher-throughput traction measurements on polyacrylamide hydrogels using gel-surface-bound fluorescent beads to permit autofocusing and automated displacement mapping, and transduction of fibroblasts with a fluorescent label to streamline cell boundary identification. Together these advances substantially improve the throughput of traction microscopy and allow us to efficiently compute the forces exerted by lung fibroblasts on substrates spanning the stiffness range present in normal and fibrotic lung tissue. Our results reveal that lung fibroblasts dramatically alter the forces they transmit to the extracellular matrix as its stiffness changes, with very low forces generated on matrices as compliant as normal lung tissue. Moreover, exogenous TGF-β1 selectively accentuates tractions on stiff matrices, mimicking fibrotic lung, but not on physiological stiffness matrices, despite equivalent changes in Smad2/3 activation. Taken together, these results demonstrate a pivotal role for matrix mechanical properties in regulating baseline and TGF-β1-stimulated contraction of lung fibroblasts and suggest that stiff fibrotic lung tissue may promote myofibroblast activation through contractility-driven events, whereas normal lung tissue compliance may protect against such feedback amplification of fibroblast activation.
Figures



Similar articles
-
Specific Features of Fibrotic Lung Fibroblasts Highly Sensitive to Fibrotic Processes Mediated via TGF-β-ERK5 Interaction.Cell Physiol Biochem. 2019;52(4):822-837. doi: 10.33594/000000057. Cell Physiol Biochem. 2019. PMID: 30946557
-
Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli.Am J Pathol. 2004 Aug;165(2):659-69. doi: 10.1016/s0002-9440(10)63330-5. Am J Pathol. 2004. PMID: 15277239 Free PMC article.
-
Nuclear factor (NF)-κB p65 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-β.Life Sci. 2015 Feb 1;122:8-14. doi: 10.1016/j.lfs.2014.11.033. Epub 2014 Dec 11. Life Sci. 2015. PMID: 25498897
-
Biomechanical Force and Cellular Stiffness in Lung Fibrosis.Am J Pathol. 2022 May;192(5):750-761. doi: 10.1016/j.ajpath.2022.02.001. Epub 2022 Feb 17. Am J Pathol. 2022. PMID: 35183510 Free PMC article. Review.
-
Traction force microscopy - Measuring the forces exerted by cells.Micron. 2021 Nov;150:103138. doi: 10.1016/j.micron.2021.103138. Epub 2021 Aug 12. Micron. 2021. PMID: 34416532 Review.
Cited by
-
A novel method for sensor-based quantification of single/multicellular force dynamics and stiffening in 3D matrices.Sci Adv. 2021 Apr 9;7(15):eabf2629. doi: 10.1126/sciadv.abf2629. Print 2021 Apr. Sci Adv. 2021. PMID: 33837084 Free PMC article.
-
Higher fibrotic content of endometriotic lesions is associated with diminished prostaglandin E2 signaling.Reprod Med Biol. 2021 Nov 1;21(1):e12423. doi: 10.1002/rmb2.12423. eCollection 2022 Jan. Reprod Med Biol. 2021. PMID: 34938147 Free PMC article.
-
TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression.Am J Physiol Cell Physiol. 2017 Mar 1;312(3):C277-C285. doi: 10.1152/ajpcell.00205.2016. Epub 2016 Nov 23. Am J Physiol Cell Physiol. 2017. PMID: 27881410 Free PMC article.
-
Arterial stiffness induces remodeling phenotypes in pulmonary artery smooth muscle cells via YAP/TAZ-mediated repression of cyclooxygenase-2.Am J Physiol Lung Cell Mol Physiol. 2017 Sep 1;313(3):L628-L647. doi: 10.1152/ajplung.00173.2017. Epub 2017 Jun 22. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28642262 Free PMC article.
-
Matrix, mesenchyme, and mechanotransduction.Ann Am Thorac Soc. 2015 Mar;12 Suppl 1(Suppl 1):S24-9. doi: 10.1513/AnnalsATS.201407-320MG. Ann Am Thorac Soc. 2015. PMID: 25830830 Free PMC article. Review.
References
-
- Ailenberg M, Tung PS, Fritz IB. Transforming growth factor-beta elicits shape changes and increases contractility of testicular peritubular cells. Biol Reprod 42: 499–509, 1990 - PubMed
-
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472, 2001 - PubMed
-
- Brown RA, Sethi KK, Gwanmesia I, Raemdonck D, Eastwood M, Mudera V. Enhanced fibroblast contraction of 3D collagen lattices and integrin expression by TGF-beta1 and -beta3: mechanoregulatory growth factors? Exp Cell Res 274: 310–322, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

