Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness
- PMID: 22659883
- PMCID: PMC3423859
- DOI: 10.1152/ajplung.00108.2012
Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness
Abstract
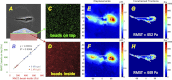
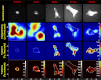
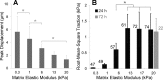
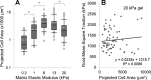
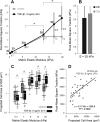
Lung fibroblast functions such as matrix remodeling and activation of latent transforming growth factor-β1 (TGF-β1) are associated with expression of the myofibroblast phenotype and are directly linked to fibroblast capacity to generate force and deform the extracellular matrix. However, the study of fibroblast force-generating capacities through methods such as traction force microscopy is hindered by low throughput and time-consuming procedures. In this study, we improved at the detail level methods for higher-throughput traction measurements on polyacrylamide hydrogels using gel-surface-bound fluorescent beads to permit autofocusing and automated displacement mapping, and transduction of fibroblasts with a fluorescent label to streamline cell boundary identification. Together these advances substantially improve the throughput of traction microscopy and allow us to efficiently compute the forces exerted by lung fibroblasts on substrates spanning the stiffness range present in normal and fibrotic lung tissue. Our results reveal that lung fibroblasts dramatically alter the forces they transmit to the extracellular matrix as its stiffness changes, with very low forces generated on matrices as compliant as normal lung tissue. Moreover, exogenous TGF-β1 selectively accentuates tractions on stiff matrices, mimicking fibrotic lung, but not on physiological stiffness matrices, despite equivalent changes in Smad2/3 activation. Taken together, these results demonstrate a pivotal role for matrix mechanical properties in regulating baseline and TGF-β1-stimulated contraction of lung fibroblasts and suggest that stiff fibrotic lung tissue may promote myofibroblast activation through contractility-driven events, whereas normal lung tissue compliance may protect against such feedback amplification of fibroblast activation.
Figures



References
-
- Ailenberg M, Tung PS, Fritz IB. Transforming growth factor-beta elicits shape changes and increases contractility of testicular peritubular cells. Biol Reprod 42: 499–509, 1990 - PubMed
-
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472, 2001 - PubMed
-
- Brown RA, Sethi KK, Gwanmesia I, Raemdonck D, Eastwood M, Mudera V. Enhanced fibroblast contraction of 3D collagen lattices and integrin expression by TGF-beta1 and -beta3: mechanoregulatory growth factors? Exp Cell Res 274: 310–322, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

