Heart repair by reprogramming non-myocytes with cardiac transcription factors
- PMID: 22660318
- PMCID: PMC3367390
- DOI: 10.1038/nature11139
Heart repair by reprogramming non-myocytes with cardiac transcription factors
Abstract
The adult mammalian heart possesses little regenerative potential following injury. Fibrosis due to activation of cardiac fibroblasts impedes cardiac regeneration and contributes to loss of contractile function, pathological remodelling and susceptibility to arrhythmias. Cardiac fibroblasts account for a majority of cells in the heart and represent a potential cellular source for restoration of cardiac function following injury through phenotypic reprogramming to a myocardial cell fate. Here we show that four transcription factors, GATA4, HAND2, MEF2C and TBX5, can cooperatively reprogram adult mouse tail-tip and cardiac fibroblasts into beating cardiac-like myocytes in vitro. Forced expression of these factors in dividing non-cardiomyocytes in mice reprograms these cells into functional cardiac-like myocytes, improves cardiac function and reduces adverse ventricular remodelling following myocardial infarction. Our results suggest a strategy for cardiac repair through reprogramming fibroblasts resident in the heart with cardiogenic transcription factors or other molecules.
Figures
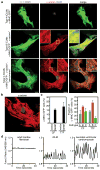
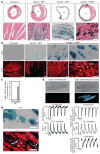

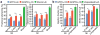

Comment in
-
Regenerative medicine: Reprogramming the injured heart.Nature. 2012 May 31;485(7400):585-6. doi: 10.1038/485585a. Nature. 2012. PMID: 22660313 No abstract available.
-
Induction of cardiomyocytes from cardiac fibroblasts.Circ Cardiovasc Genet. 2012 Aug 1;5(4):481-2. doi: 10.1161/CIRCGENETICS.112.964288. Circ Cardiovasc Genet. 2012. PMID: 22896018 No abstract available.
-
Reprogrammed cardiac fibroblasts to the rescue of heart failure.Circ Res. 2012 Sep 14;111(7):831-2. doi: 10.1161/CIRCRESAHA.112.279745. Circ Res. 2012. PMID: 22982872 No abstract available.
References
-
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. - PubMed
-
- Yacoub M, Suzuki K, Rosenthal N. The future of regenerative therapy in patients with chronic heart failure. Nat Clin Pract Cardiovasc Med. 2006;3 (Suppl 1):S133–135. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 HL077439/HL/NHLBI NIH HHS/United States
- R01 AR040339/AR/NIAMS NIH HHS/United States
- R01 HL111665/HL/NHLBI NIH HHS/United States
- P50 HL061033/HL/NHLBI NIH HHS/United States
- S10 RR019137/RR/NCRR NIH HHS/United States
- R01 HL074257/HL/NHLBI NIH HHS/United States
- K08 HL111420/HL/NHLBI NIH HHS/United States
- R01 HL093039/HL/NHLBI NIH HHS/United States
- K99 HL114738/HL/NHLBI NIH HHS/United States
- R37 HL053351/HL/NHLBI NIH HHS/United States
- R01 HL083371/HL/NHLBI NIH HHS/United States
- R01 HL061544/HL/NHLBI NIH HHS/United States
- R01 HL053351/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

