AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress
- PMID: 22660331
- PMCID: PMC3607316
- DOI: 10.1038/nature11066
AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress
Abstract
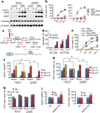
Overcoming metabolic stress is a critical step for solid tumour growth. However, the underlying mechanisms of cell death and survival under metabolic stress are not well understood. A key signalling pathway involved in metabolic adaptation is the liver kinase B1 (LKB1)-AMP-activated protein kinase (AMPK) pathway. Energy stress conditions that decrease intracellular ATP levels below a certain level promote AMPK activation by LKB1. Previous studies showed that LKB1-deficient or AMPK-deficient cells are resistant to oncogenic transformation and tumorigenesis, possibly because of the function of AMPK in metabolic adaptation. However, the mechanisms by which AMPK promotes metabolic adaptation in tumour cells are not fully understood. Here we show that AMPK activation, during energy stress, prolongs cell survival by redox regulation. Under these conditions, NADPH generation by the pentose phosphate pathway is impaired, but AMPK induces alternative routes to maintain NADPH and inhibit cell death. The inhibition of the acetyl-CoA carboxylases ACC1 and ACC2 by AMPK maintains NADPH levels by decreasing NADPH consumption in fatty-acid synthesis and increasing NADPH generation by means of fatty-acid oxidation. Knockdown of either ACC1 or ACC2 compensates for AMPK activation and facilitates anchorage-independent growth and solid tumour formation in vivo, whereas the activation of ACC1 or ACC2 attenuates these processes. Thus AMPK, in addition to its function in ATP homeostasis, has a key function in NADPH maintenance, which is critical for cancer cell survival under energy stress conditions, such as glucose limitations, anchorage-independent growth and solid tumour formation in vivo.
Figures
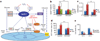


Comment in
-
Cancer metabolism: Tumour friend or foe.Nature. 2012 May 31;485(7400):590-1. doi: 10.1038/485590a. Nature. 2012. PMID: 22660317 No abstract available.
References
-
- Folkman J. Angiogenesis and apoptosis. Semin. Cancer Biol. 2003;13:159–167. - PubMed
-
- Bardeesy N, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. - PubMed
-
- Kato K, et al. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

