Coordinated regulation of neuronal mRNA steady-state levels through developmentally controlled intron retention
- PMID: 22661231
- PMCID: PMC3371409
- DOI: 10.1101/gad.188037.112
Coordinated regulation of neuronal mRNA steady-state levels through developmentally controlled intron retention
Abstract
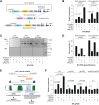
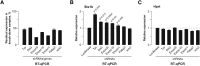
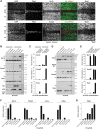
Differentiated cells acquire unique structural and functional traits through coordinated expression of lineage-specific genes. An extensive battery of genes encoding components of the synaptic transmission machinery and specialized cytoskeletal proteins is activated during neurogenesis, but the underlying regulation is not well understood. Here we show that genes encoding critical presynaptic proteins are transcribed at a detectable level in both neurons and nonneuronal cells. However, in nonneuronal cells, the splicing of 3'-terminal introns within these genes is repressed by the polypyrimidine tract-binding protein (Ptbp1). This inhibits the export of incompletely spliced mRNAs to the cytoplasm and triggers their nuclear degradation. Clearance of these intron-containing transcripts occurs independently of the nonsense-mediated decay (NMD) pathway but requires components of the nuclear RNA surveillance machinery, including the nuclear pore-associated protein Tpr and the exosome complex. When Ptbp1 expression decreases during neuronal differentiation, the regulated introns are spliced out, thus allowing the accumulation of translation-competent mRNAs in the cytoplasm. We propose that this mechanism counters ectopic and precocious expression of functionally linked neuron-specific genes and ensures their coherent activation in the appropriate developmental context.
Figures




References
-
- Ameur A, Zaghlool A, Halvardson J, Wetterbom A, Gyllensten U, Cavelier L, Feuk L 2011. Total RNA sequencing reveals nascent transcription and widespread co-transcriptional splicing in the human brain. Nat Struct Mol Biol 18: 1435–1440 - PubMed
-
- Averbeck N, Sunder S, Sample N, Wise JA, Leatherwood J 2005. Negative control contributes to an extensive program of meiotic splicing in fission yeast. Mol Cell 18: 491–498 - PubMed
-
- Ballas N, Mandel G 2005. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol 15: 500–506 - PubMed
-
- Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ 2010. Deciphering the splicing code. Nature 465: 53–59 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
