Evidence for premature aging due to oxidative stress in iPSCs from Cockayne syndrome
- PMID: 22661500
- PMCID: PMC3412382
- DOI: 10.1093/hmg/dds211
Evidence for premature aging due to oxidative stress in iPSCs from Cockayne syndrome
Abstract
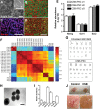
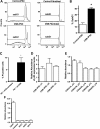
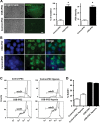
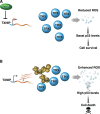
Cockayne syndrome (CS) is a human premature aging disorder associated with neurological and developmental abnormalities, caused by mutations mainly in the CS group B gene (ERCC6). At the molecular level, CS is characterized by a deficiency in the transcription-couple DNA repair pathway. To understand the role of this molecular pathway in a pluripotent cell and the impact of CSB mutation during human cellular development, we generated induced pluripotent stem cells (iPSCs) from CSB skin fibroblasts (CSB-iPSC). Here, we showed that the lack of functional CSB does not represent a barrier to genetic reprogramming. However, iPSCs derived from CSB patient's fibroblasts exhibited elevated cell death rate and higher reactive oxygen species (ROS) production. Moreover, these cellular phenotypes were accompanied by an up-regulation of TXNIP and TP53 transcriptional expression. Our findings suggest that CSB modulates cell viability in pluripotent stem cells, regulating the expression of TP53 and TXNIP and ROS production.
Figures
References
-
- Weidenheim K.M., Dickson D.W., Rapin I. Neuropathology of Cockayne syndrome: evidence for impaired development, premature aging, and neurodegeneration. Mech. Ageing Dev. 2009;130:619–636. - PubMed
-
- Laugel V., Dalloz C., Durand M., Sauvanaud F., Kristensen U., Vincent M.C., Pasquier L., Odent S., Cormier-Daire V., Gener B., et al. Molecular analysis of mutations in the CSB (ERCC6) gene in patients with Cockayne syndrome. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum. Mutat. 2010;31:113–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

