A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP
- PMID: 22661686
- PMCID: PMC3415487
- DOI: 10.1128/JB.00379-12
A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP
Abstract
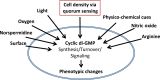
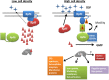
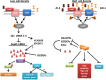
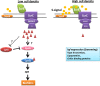
Bacteria sense and respond to environmental cues to control important developmental processes. Two widely conserved and important strategies that bacteria employ to sense changes in population density and local environmental conditions are quorum sensing (QS) and cyclic di-GMP (c-di-GMP) signaling, respectively. The importance of these pathways in controlling a broad variety of functions, including virulence, biofilm formation, and motility, has been recognized in many species. Recent research has shown that these pathways are intricately intertwined. Here we review the regulatory connections between QS and c-di-GMP signaling. We propose that the integration of QS with c-di-GMP allows bacteria to assimilate information about the local bacterial population density with other physicochemical environmental signals within the broader c-di-GMP signaling network.
Figures


References
-
- Andrade MO, et al. 2006. The HD-GYP domain of RpfG mediates a direct linkage between the Rpf quorum-sensing pathway and a subset of diguanylate cyclase proteins in the phytopathogen Xanthomonas axonopodis pv citri. Mol. Microbiol. 62:537–551 - PubMed
-
- Barber CE, et al. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555–566 - PubMed
-
- Beyhan S, Bilecen K, Salama SR, Casper-Lindley C, Yildiz FH. 2007. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 189:388–402 doi:10.1128/JB.00981-06 - DOI - PMC - PubMed
-
- Beyhan S, Tischler AD, Camilli A, Yildiz FH. 2006. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J. Bacteriol. 188:3600–3613 doi:10.1128/JB.188.10.3600-3613.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

