Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins
- PMID: 22661688
- PMCID: PMC3415520
- DOI: 10.1128/JB.00011-12
Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins
Abstract
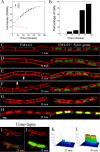
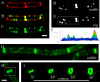
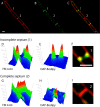
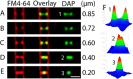
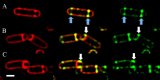
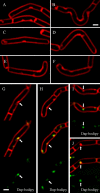
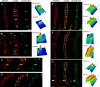
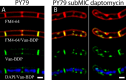
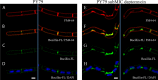
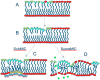
Daptomycin is a lipopeptide antibiotic used clinically for the treatment of certain types of Gram-positive infections, including those caused by methicillin-resistant Staphylococcus aureus (MRSA). Details of the mechanism of action of daptomycin continue to be elucidated, particularly the question of whether daptomycin acts on the cell membrane, the cell wall, or both. Here, we use fluorescence microscopy to directly visualize the interaction of daptomycin with the model Gram-positive bacterium Bacillus subtilis. We show that the first observable cellular effects are the formation of membrane distortions (patches of membrane) that precede cell death by more than 30 min. Membrane patches are able to recruit the essential cell division protein DivIVA. Recruitment of DivIVA correlates with membrane defects and changes in cell morphology, suggesting a localized alteration in the activity of enzymes involved in cell wall synthesis that could account for previously described effects of daptomycin on cell wall morphology and septation. Membrane defects colocalize with fluorescently labeled daptomycin, DivIVA, and fluorescent reporters of peptidoglycan biogenesis (Bocillin FL and BODIPY FL-vancomycin), suggesting that daptomycin plays a direct role in these events. Our results support a mechanism for daptomycin with a primary effect on cell membranes that in turn redirects the localization of proteins involved in cell division and cell wall synthesis, causing dramatic cell wall and membrane defects, which may ultimately lead to a breach in the cell membrane and cell death. These results help resolve the longstanding questions regarding the mechanism of action of this important class of antibiotics.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

