Impaired natural killer cell self-education and "missing-self" responses in Ly49-deficient mice
- PMID: 22661698
- PMCID: PMC3976224
- DOI: 10.1182/blood-2012-02-408732
Impaired natural killer cell self-education and "missing-self" responses in Ly49-deficient mice
Erratum in
- Blood. 2013 Oct 3;122(14):2525
Abstract
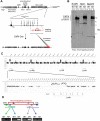
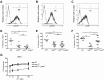
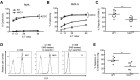
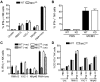
Ly49-mediated recognition of MHC-I molecules on host cells is considered vital for natural killer (NK)-cell regulation and education; however, gene-deficient animal models are lacking because of the difficulty in deleting this large multigene family. Here, we describe NK gene complex knockdown (NKC(KD)) mice that lack expression of Ly49 and related MHC-I receptors on most NK cells. NKC(KD) NK cells exhibit defective killing of MHC-I-deficient, but otherwise normal, target cells, resulting in defective rejection by NKC(KD) mice of transplants from various types of MHC-I-deficient mice. Self-MHC-I immunosurveillance by NK cells in NKC(KD) mice can be rescued by self-MHC-I-specific Ly49 transgenes. Although NKC(KD) mice display defective recognition of MHC-I-deficient tumor cells, resulting in decreased in vivo tumor cell clearance, NKG2D- or antibody-dependent cell-mediated cytotoxicity-induced tumor cell cytotoxicity and cytokine production induced by activation receptors was efficient in Ly49-deficient NK cells, suggesting MHC-I education of NK cells is a single facet regulating their total potential. These results provide direct genetic evidence that Ly49 expression is necessary for NK-cell education to self-MHC-I molecules and that the absence of these receptors leads to loss of MHC-I-dependent "missing-self" immunosurveillance by NK cells.
Figures



References
-
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. - PubMed
-
- Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6(6):399–409. - PubMed
-
- Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–678. - PubMed
-
- Carlyle JR, Mesci A, Fine JH, et al. Evolution of the Ly49 and Nkrp1 recognition systems. Semin Immunol. 2008;20(6):321–330. - PubMed
-
- Makrigiannis AP, Patel D, Goulet ML, Dewar K, Anderson SK. Direct sequence comparison of two divergent class I MHC natural killer cell receptor haplotypes. Genes Immun. 2005;6(2):71–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

