Ultrasmall superparamagnetic iron oxide (USPIO)-based liposomes as magnetic resonance imaging probes
- PMID: 22661890
- PMCID: PMC3357980
- DOI: 10.2147/IJN.S30617
Ultrasmall superparamagnetic iron oxide (USPIO)-based liposomes as magnetic resonance imaging probes
Abstract
Background: Magnetic liposomes (MLs) are phospholipid vesicles that encapsulate magnetic and/or paramagnetic nanoparticles. They are applied as contrast agents for magnetic resonance imaging (MRI). MLs have an advantage over free magnetic nanocores, in that various functional groups can be attached to the surface of liposomes for ligand-specific targeting. We have synthesized PEG-coated sterically-stabilized magnetic liposomes (sMLs) containing ultrasmall superparamagnetic iron oxides (USPIOs) with the aim of generating stable liposomal carriers equipped with a high payload of USPIOs for enhanced MRI contrast.
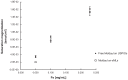
Methods: Regarding iron oxide nanoparticles, we have applied two different commercially available surface-coated USPIOs; sMLs synthesized and loaded with USPIOs were compared in terms of magnetization and colloidal stability. The average diameter size, morphology, phospholipid membrane fluidity, and the iron content of the sMLs were determined by dynamic light scattering (DLS), transmission electron microscopy (TEM), fluorescence polarization, and absorption spectroscopy, respectively. A colorimetric assay using potassium thiocyanate (KSCN) was performed to evaluate the encapsulation efficiency (EE%) to express the amount of iron enclosed into a liposome. Subsequently, MRI measurements were carried out in vitro in agarose gel phantoms to evaluate the signal enhancement on T1- and T2-weighted sequences of sMLs. To monitor the biodistribution and the clearance of the particles over time in vivo, sMLs were injected in wild type mice.
Results: DLS revealed a mean particle diameter of sMLs in the range between 100 and 200 nm, as confirmed by TEM. An effective iron oxide loading was achieved just for one type of USPIO, with an EE% between 74% and 92%, depending on the initial Fe concentration (being higher for lower amounts of Fe). MRI measurements demonstrated the applicability of these nanostructures as MRI probes.
Conclusion: Our results show that the development of sMLs is strictly dependent on the physicochemical characteristics of the nanocores. Once established, sMLs can be further modified to enable noninvasive targeted molecular imaging.
Keywords: MRI contrast agent; biodistribution; fluorescence polarization; magnetic liposomes.
Figures






References
-
- Torchilin VP. Liposomes as delivery agents for medical imaging. Mol Med Today. 1996;2(6):242–249. - PubMed
-
- Williams DF. Williams Dictionary of Biomaterials. Liverpool: University Press; 1999.
-
- Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19(1):142–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

