Cardiac complications associated with trastuzumab in the setting of adjuvant chemotherapy for breast cancer overexpressing human epidermal growth factor receptor type 2 - a prospective study
- PMID: 22661994
- PMCID: PMC3361034
- DOI: 10.5114/aoms.2012.28549
Cardiac complications associated with trastuzumab in the setting of adjuvant chemotherapy for breast cancer overexpressing human epidermal growth factor receptor type 2 - a prospective study
Abstract
Introduction: Trastuzumab, a recombinant humanized monoclonal antibody, is targeted against the external domain of the human epidermal growth factor receptor type 2 (HER2). It improves efficacy of HER2-positive breast cancer treatment. The authors present their experience with patients (pts) treated with trastuzumab in the aspects of cardiac complications.
Material and methods: We observed prospectively 253 women with early positive HER2 breast cancer treated with trastuzumab. Assessment of cardiovascular status, ECG and echocardiography was performed initially and every 3 months until 6(th) month during follow-up.
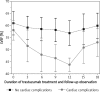
Results: Cardiac complications developed in 52 pts (20.55%) and included: asymptomatic left ventricle dysfunction (43), symptomatic heart failure (6), new asymptomatic LBBB (1); new negative T-waves in ECG (2). There was a progressive decline in left ventricular ejection fraction (LVEF) during treatment. It was more enhanced in pts with cardiac complications. Following trastuzumab termination/discontinuation LVEF increased but at month 18 still remained significantly lower than initially in both groups (61.07 ±4.84 vs. 59.97 ±5.23 - no cardiac complications; p < 0.05; 58.14 ±4.08% vs. 53.08 ±5.74% - cardiac complications; p < 0.05). During 6-month follow-up 33 out of 46 pts experienced an improvement in left ventricular status. In 13 pts in whom trastuzumab was discontinued, it was restarted; 6 of them successfully completed total therapy. Univariate analysis revealed no association between any cardiovascular risk factor and the development of cardiotoxicity.
Conclusions: One out of five treated patients discontinues trastuzumab in an adjuvant setting due to cardiac complications. LV dysfunction is the most frequent. Routine cardiac monitoring should be obligatory.
Keywords: HER2 overexpression; breast cancer; cardiotoxicity; trastuzumab; trastuzumab-related cardiomyopathy.
Figures
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Force T, Krause DS. Van Etten. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–44. - PubMed
-
- Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: amilestone in the treatment of HER-2 positive early. Oncologist. 2006;11(Suppl 1):4–12. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous