Development and validation of a low cost blood filtration element separating plasma from undiluted whole blood
- PMID: 22662072
- PMCID: PMC3365324
- DOI: 10.1063/1.3672188
Development and validation of a low cost blood filtration element separating plasma from undiluted whole blood
Abstract
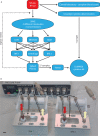
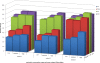
Clinical point of care testing often needs plasma instead of whole blood. As centrifugation is labor intensive and not always accessible, filtration is a more appropriate separation technique. The complexity of whole blood is such that there is still no commercially available filtration system capable of separating small sample volumes (10-100 μl) at the point of care. The microfluidics research in blood filtration is very active but to date nobody has validated a low cost device that simultaneously filtrates small samples of whole blood and reproducibly recovers clinically relevant biomarkers, and all this in a limited amount of time with undiluted raw samples. In this paper, we show first that plasma filtration from undiluted whole blood is feasible and reproducible in a low-cost microfluidic device. This novel microfluidic blood filtration element (BFE) extracts 12 μl of plasma from 100 μl of whole blood in less than 10 min. Then, we demonstrate that our device is valid for clinical studies by measuring the adsorption of interleukins through our system. This adsorption is reproducible for interleukins IL6, IL8, and IL10 but not for TNFα. Hence, our BFE is valid for clinical diagnostics with simple calibration prior to performing any measurement.
Figures

References
-
- Kemmler M., Koger B., Sulz G., Sauer U., Schleicher E., Preininger C., and Brandenburg A., Sens. Actuators B 139, 44 (2009).10.1016/j.snb.2008.08.043 - DOI
-
- Li N., Kamei D. T., and Ho C.-M., in Proceedings of the 2nd IEEE Conference on Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, January 16–19, 2007.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
