Reduced cortisol and metabolic responses of thin ewes to an acute cold challenge in mid-pregnancy: implications for animal physiology and welfare
- PMID: 22662144
- PMCID: PMC3360704
- DOI: 10.1371/journal.pone.0037315
Reduced cortisol and metabolic responses of thin ewes to an acute cold challenge in mid-pregnancy: implications for animal physiology and welfare
Abstract
Background: Low food availability leading to reductions in Body Condition Score (BCS; 0 indicates emaciation and 5 obesity) in sheep often coincides with low temperatures associated with the onset of winter in New Zealand. The ability to adapt to reductions in environmental temperature may be impaired in animals with low BCS, in particular during pregnancy when metabolic demand is higher. Here we assess whether BCS affects a pregnant animal's ability to cope with cold challenges.
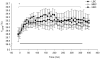
Methods: Eighteen pregnant ewes with a BCS of 2.7±0.1 were fed to attain low (LBC: BCS2.3±0.1), medium (MBC: BCS3.2±0.2) or high BCS (HBC: BCS3.6±0.2). Shorn ewes were exposed to a 6-h acute cold challenge in a climate-controlled room (wet and windy conditions, 4.4±0.1°C) in mid-pregnancy. Blood samples were collected during the BCS change phase, acute cold challenge and recovery phase.
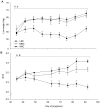
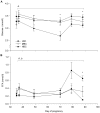
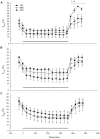
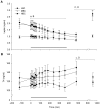
Results: During the BCS change phase, plasma glucose and leptin concentrations declined while free fatty acids (FFA) increased in LBC compared to MBC (P<0.01, P<0.01 and P<0.05, respectively) and HBC ewes (P<0.05, P<0.01 and P<0.01, respectively). During the cold challenge, plasma cortisol concentrations were lower in LBC than MBC (P<0.05) and HBC ewes (P<0.05), and FFA and insulin concentrations were lower in LBC than HBC ewes (P<0.05 and P<0.001, respectively). Leptin concentrations declined in MBC and HBC ewes while remaining unchanged in LBC ewes (P<0.01). Glucose concentrations and internal body temperature (T(core)) increased in all treatments, although peak T(core) tended to be higher in HBC ewes (P<0.1). During the recovery phase, T4 concentrations were lower in LBC ewes (P<0.05).
Conclusion: Even though all ewes were able to increase T(core) and mobilize glucose, low BCS animals had considerably reduced cortisol and metabolic responses to a cold challenge in mid-pregnancy, suggesting that their ability to adapt to cold challenges through some of the expected pathways was reduced.
Conflict of interest statement
Figures




References
-
- Kenyon PR, Morris ST, Revell DK, McCutcheon SN. Shearing during pregnancy - review of a policy to increase birthweight and survival of lambs in New Zealand pastoral farming systems. New Zeal Vet J. 2003;51:200–207. - PubMed
-
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. - PubMed
-
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. - PubMed
-
- Brunton PJ, Russell JA, Douglas AJ. Adaptive Responses of the Maternal Hypothalamic-Pituitary-Adrenal Axis during Pregnancy and Lactation. J Neuroendocinol. 2008;20:764–776. - PubMed
-
- Turner AI, Keating CL, Tilbrook AJ. Sex Differences and the Role of Sex Steroids in Sympatho-Adrenal Medullary System and Hypothalamo-Pituitary Adrenal Axis Responses to Stress. In: Kahn SM, editor. Sex steroids. Rijeka, Croatia: InTech; 2012. pp. 115–136.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

