MRI tracking of FePro labeled fresh and cryopreserved long term in vitro expanded human cord blood AC133+ endothelial progenitor cells in rat glioma
- PMID: 22662174
- PMCID: PMC3360770
- DOI: 10.1371/journal.pone.0037577
MRI tracking of FePro labeled fresh and cryopreserved long term in vitro expanded human cord blood AC133+ endothelial progenitor cells in rat glioma
Abstract
Background: Endothelial progenitors cells (EPCs) are important for the development of cell therapies for various diseases. However, the major obstacles in developing such therapies are low quantities of EPCs that can be generated from the patient and the lack of adequate non-invasive imaging approach for in vivo monitoring of transplanted cells. The objective of this project was to determine the ability of cord blood (CB) AC133+ EPCs to differentiate, in vitro and in vivo, toward mature endothelial cells (ECs) after long term in vitro expansion and cryopreservation and to use magnetic resonance imaging (MRI) to assess the in vivo migratory potential of ex vivo expanded and cryopreserved CB AC133+ EPCs in an orthotopic glioma rat model.
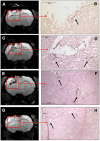
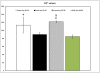
Materials, methods and results: The primary CB AC133+ EPC culture contained mainly EPCs and long term in vitro conditions facilitated the maintenance of these cells in a state of commitment toward endothelial lineage. At days 15-20 and 25-30 of the primary culture, the cells were labeled with FePro and cryopreserved for a few weeks. Cryopreserved cells were thawed and in vitro differentiated or i.v. administered to glioma bearing rats. Different groups of rats also received long-term cultured, magnetically labeled fresh EPCs and both groups of animals underwent MRI 7 days after i.v. administration of EPCs. Fluorescent microscopy showed that in vitro differentiation of EPCs was not affected by FePro labeling and cryopreservation. MRI analysis demonstrated that in vivo accumulation of previously cryopreserved transplanted cells resulted in significantly higher R2 and R2* values indicating a higher rate of migration and incorporation into tumor neovascularization of previously cryopreserved CB AC133+ EPCs to glioma sites, compared to non-cryopreserved cells.
Conclusion: Magnetically labeled CB EPCs can be in vitro expanded and cryopreserved for future use as MRI probes for monitoring the migration and incorporation to the sites of neovascularization.
Conflict of interest statement
Figures



References
-
- Scott SM, Barth MG, Gaddy LR, Ahl ET The role of circulating cells in the healing of vascular prostheses. J Vasc Surg. 1994;19:585–593. - PubMed
-
- Solovey A, Lin Y, Browne P, Choong S, Wayner E, et al. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. - PubMed
-
- Sowemimo-Coker SO, Meiselman HJ, Francis RB Increased circulating endothelial cells in sickle cell crisis. Am J Hematol. 1989;31:263–265. - PubMed
-
- Hladovec J. Circulating endothelial cells as a sign of vessel wall lesions. Physiol Bohemoslov. 1978;27:140–144. - PubMed
-
- Hladovec J, Prerovsky I, Stanek V, Fabian J. Circulating endothelial cells in acute myocardial infarction and angina pectoris. Klin Wochenschr. 1978;56:1033–1036. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

