Functional analysis of a breast cancer-associated mutation in the intracellular domain of the metalloprotease ADAM12
- PMID: 22662180
- PMCID: PMC3360752
- DOI: 10.1371/journal.pone.0037628
Functional analysis of a breast cancer-associated mutation in the intracellular domain of the metalloprotease ADAM12
Abstract
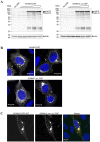
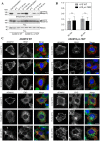
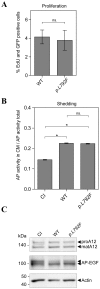
A recently identified breast cancer-associated mutation in the metalloprotease ADAM12 alters a potential dileucine trafficking signal, which could affect protein processing and cellular localization. ADAM12 belongs to the group of A Disintegrin And Metalloproteases (ADAMs), which are typically membrane-associated proteins involved in ectodomain shedding, cell-adhesion, and signaling. ADAM12 as well as several members of the ADAM family are over-expressed in various cancers, correlating with disease stage. Three breast cancer-associated somatic mutations were previously identified in ADAM12, and two of these, one in the metalloprotease domain and another in the disintegrin domain, were investigated and found to result in protein misfolding, retention in the secretory pathway, and failure of zymogen maturation. The third mutation, p.L792F in the ADAM12 cytoplasmic tail, was not investigated, but is potentially significant given its location within a di-leucine motif, which is recognized as a potential cellular trafficking signal. The present study was motivated both by the potential relevance of this documented mutation to cancer, as well as for determining the role of the di-leucine motif in ADAM12 trafficking. Expression of ADAM12 p.L792F in mammalian cells demonstrated quantitatively similar expression levels and zymogen maturation as wild-type (WT) ADAM12, as well as comparable cellular localizations. A cell surface biotinylation assay demonstrated that cell surface levels of ADAM12 WT and ADAM12 p.L792F were similar and that internalization of the mutant occurred at the same rate and extent as for ADAM12 WT. Moreover, functional analysis revealed no differences in cell proliferation or ectodomain shedding of epidermal growth factor (EGF), a known ADAM12 substrate between WT and mutant ADAM12. These data suggest that the ADAM12 p.L792F mutation is unlikely to be a driver (cancer causing)-mutation in breast cancer.
Conflict of interest statement
Figures
Similar articles
-
Phenotypic diversity of breast cancer-related mutations in metalloproteinase-disintegrin ADAM12.PLoS One. 2014 Mar 20;9(3):e92536. doi: 10.1371/journal.pone.0092536. eCollection 2014. PLoS One. 2014. PMID: 24651654 Free PMC article.
-
Breast cancer-associated mutations in metalloprotease disintegrin ADAM12 interfere with the intracellular trafficking and processing of the protein.Int J Cancer. 2008 Jun 1;122(11):2634-40. doi: 10.1002/ijc.23405. Int J Cancer. 2008. PMID: 18241035 Free PMC article.
-
Cell-surface metalloprotease ADAM12 is internalized by a clathrin- and Grb2-dependent mechanism.Traffic. 2012 Nov;13(11):1532-46. doi: 10.1111/j.1600-0854.2012.01405.x. Epub 2012 Sep 10. Traffic. 2012. PMID: 22882974
-
Cellular roles of ADAM12 in health and disease.Int J Biochem Cell Biol. 2008;40(9):1685-702. doi: 10.1016/j.biocel.2008.01.025. Epub 2008 Feb 1. Int J Biochem Cell Biol. 2008. PMID: 18342566 Review.
-
Targeting ADAM12 in human disease: head, body or tail?Curr Pharm Des. 2009;15(20):2300-10. doi: 10.2174/138161209788682389. Curr Pharm Des. 2009. PMID: 19601832 Review.
Cited by
-
An essential role of metalloprotease-disintegrin ADAM12 in triple-negative breast cancer.Breast Cancer Res Treat. 2012 Oct;135(3):759-69. doi: 10.1007/s10549-012-2220-4. Epub 2012 Aug 29. Breast Cancer Res Treat. 2012. PMID: 22926263 Free PMC article.
-
Phenotypic diversity of breast cancer-related mutations in metalloproteinase-disintegrin ADAM12.PLoS One. 2014 Mar 20;9(3):e92536. doi: 10.1371/journal.pone.0092536. eCollection 2014. PLoS One. 2014. PMID: 24651654 Free PMC article.
-
ADAM12-L is a direct target of the miR-29 and miR-200 families in breast cancer.BMC Cancer. 2015 Mar 4;15:93. doi: 10.1186/s12885-015-1108-1. BMC Cancer. 2015. PMID: 25886595 Free PMC article.
References
-
- Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

