Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia
- PMID: 22662206
- PMCID: PMC3360607
- DOI: 10.1371/journal.pone.0037746
Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia
Abstract
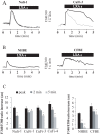
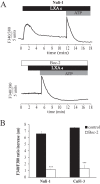
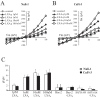
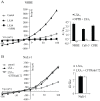
Cystic Fibrosis (CF) is a genetic disease characterised by a deficit in epithelial Cl(-) secretion which in the lung leads to airway dehydration and a reduced Airway Surface Liquid (ASL) height. The endogenous lipoxin LXA(4) is a member of the newly identified eicosanoids playing a key role in ending the inflammatory process. Levels of LXA(4) are reported to be decreased in the airways of patients with CF. We have previously shown that in normal human bronchial epithelial cells, LXA(4) produced a rapid and transient increase in intracellular Ca(2+). We have investigated, the effect of LXA(4) on Cl(-) secretion and the functional consequences on ASL generation in bronchial epithelial cells obtained from CF and non-CF patient biopsies and in bronchial epithelial cell lines. We found that LXA(4) stimulated a rapid intracellular Ca(2+) increase in all of the different CF bronchial epithelial cells tested. In non-CF and CF bronchial epithelia, LXA(4) stimulated whole-cell Cl(-) currents which were inhibited by NPPB (calcium-activated Cl(-) channel inhibitor), BAPTA-AM (chelator of intracellular Ca(2+)) but not by CFTRinh-172 (CFTR inhibitor). We found, using confocal imaging, that LXA(4) increased the ASL height in non-CF and in CF airway bronchial epithelia. The LXA(4) effect on ASL height was sensitive to bumetanide, an inhibitor of transepithelial Cl(-) secretion. The LXA(4) stimulation of intracellular Ca(2+), whole-cell Cl(-) currents, conductances and ASL height were inhibited by Boc-2, a specific antagonist of the ALX/FPR2 receptor. Our results provide, for the first time, evidence for a novel role of LXA(4) in the stimulation of intracellular Ca(2+) signalling leading to Ca(2+)-activated Cl(-) secretion and enhanced ASL height in non-CF and CF bronchial epithelia.
Conflict of interest statement
Figures

References
-
- Boat TF, Cheng PW. Epithelial cell dysfunction in cystic fibrosis: implications for airways disease. Acta Paediatr Scand Suppl 363: 25–29; discussion. 1989;29–30 - PubMed
-
- Mickle JE, Macek M, Fulmer-Smentek SB, Egan MM, Schwiebert E, et al. A mutation in the cystic fibrosis transmembrane conductance regulator gene associated with elevated sweat chloride concentrations in the absence of cystic fibrosis. Hum Mol Genet. 1998;7:729–735. - PubMed
-
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. - PubMed
-
- Karp CL, Flick LM, Park KW, Softic S, Greer TM, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. - PubMed
-
- Serhan CN, Takano T, Gronert K, Chiang N, Clish CB. Lipoxin and aspirin-triggered 15-epi-lipoxin cellular interactions anti-inflammatory lipid mediators. Clin Chem Lab Med. 1999;37:299–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

