Proton Coupled Electron Transfer and Redox Active Tyrosines: Structure and Function of the Tyrosyl Radicals in Ribonucleotide Reductase and Photosystem II
- PMID: 22662289
- PMCID: PMC3362996
- DOI: 10.1021/jz2014117
Proton Coupled Electron Transfer and Redox Active Tyrosines: Structure and Function of the Tyrosyl Radicals in Ribonucleotide Reductase and Photosystem II
Abstract
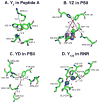
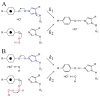
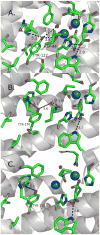
Proton coupled electron transfer (PCET) reactions are important in many biological processes. Tyrosine oxidation/reduction can play a critical role in facilitating these reactions. Two examples are photosystem II (PSII) and ribonucleotide reductase (RNR). RNR is essential in DNA synthesis in all organisms. In E. coli RNR, a tyrosyl radical, Y122(•), is required as a radical initiator. Photosystem II (PSII) generates molecular oxygen from water. In PSII, an essential tyrosyl radical, YZ(•), oxidizes the oxygen evolving center. However, the mechanisms, by which the extraordinary oxidizing power of the tyrosyl radical is controlled, are not well understood. This is due to the difficulty in acquiring high-resolution structural information about the radical state. Spectroscopic approaches, such as EPR and UV resonance Raman (UVRR), can give new information. Here, we discuss EPR studies of PCET and the PSII YZ radical. We also present UVRR results, which support the conclusion that Y122 undergoes an alteration in ring and backbone dihedral angle when it is oxidized. This conformational change results in a loss of hydrogen bonding to the phenolic oxygen. Our analysis suggests that access of water is an important factor in determining tyrosyl radical lifetime and function. TOC graphic.
Figures





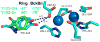
References
-
- Stubbe J. Ribonucleotide Reductases: Amazing and Confusing. J Biol Chem. 1990;265:5329–5332. - PubMed
-
- Boerner RJ, Barry BA. Isotopic Labeling and EPR Spectroscopy Show that a Tyrosine Residue is the Terminal Electron Donor, Z, in Manganese-depleted Photosystem II Preparations. J Biol Chem. 1993;268:17151–17154. - PubMed
-
- Kulmacz RJ, Ren Y, Tsai AL, Palmer G. Prostaglandin H synthase: Spectroscopic Studies of the Interaction with Hydroperoxides and with Indomethacin. Biochemistry. 1990;29:8760–8771. - PubMed
-
- Whittaker MM, Whittaker JW. Tyrosine-derived Free Radical in Apogalactose Oxidase. J Biol Chem. 1990;265:9610–9613. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

