Small-molecule inhibitors of the LEDGF/p75 binding site of integrase block HIV replication and modulate integrase multimerization
- PMID: 22664975
- PMCID: PMC3421592
- DOI: 10.1128/AAC.00717-12
Small-molecule inhibitors of the LEDGF/p75 binding site of integrase block HIV replication and modulate integrase multimerization
Abstract
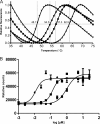
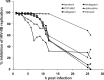
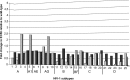
Targeting the HIV integrase (HIV IN) is a clinically validated approach for designing novel anti-HIV therapies. We have previously described the discovery of a novel class of integration inhibitors, 2-(quinolin-3-yl)acetic acid derivatives, blocking HIV replication at a low micromolar concentration through binding in the LEDGF/p75 binding pocket of HIV integrase, hence referred to as LEDGINs. Here we report the detailed characterization of their mode of action. The design of novel and more potent analogues with nanomolar activity enabled full virological evaluation and a profound mechanistic study. As allosteric inhibitors, LEDGINs bind to the LEDGF/p75 binding pocket in integrase, thereby blocking the interaction with LEDGF/p75 and interfering indirectly with the catalytic activity of integrase. Detailed mechanism-of-action studies reveal that the allosteric mode of inhibition is likely caused by an effect on HIV-1 integrase oligomerization. The multimodal inhibition by LEDGINs results in a block in HIV integration and in a replication deficiency of progeny virus. The allosteric nature of LEDGINs leads to synergy in combination with the clinically approved active site HIV IN strand transfer inhibitor (INSTI) raltegravir, and cross-resistance profiling proves the distinct mode of action of LEDGINs and INSTIs. The allosteric nature of inhibition and compatibility with INSTIs underline an interest in further (clinical) development of LEDGINs.
Figures




References
-
- Blair WS, et al. 2010. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 6:e1001220 doi:10.1371/journal.ppat.1001220 - DOI - PMC - PubMed
-
- Bruno R, Sacchi P, Filice G. 2003. Didanosine-ribavirin combination: synergistic combination in vitro, but high potential risk of toxicity in vivo. AIDS 17:2674–2675 - PubMed
-
- Busschots K, et al. 2005. The interaction of LEDGF/p75 with integrase is lentivirus-specific and promotes DNA binding. J. Biol. Chem. 280:17841–17847 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

