Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model
- PMID: 22665162
- PMCID: PMC3524267
- DOI: 10.22203/ecm.v023a31
Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model
Abstract
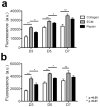
Peripheral artery disease (PAD) currently affects approximately 27 million patients in Europe and North America, and if untreated, may progress to the stage of critical limb ischemia (CLI), which has implications for amputation and potential mortality. Unfortunately, few therapies exist for treating the ischemic skeletal muscle in these conditions. Biomaterials have been used to increase cell transplant survival as well as deliver growth factors to treat limb ischemia; however, existing materials do not mimic the native skeletal muscle microenvironment they are intended to treat. Furthermore, no therapies involving biomaterials alone have been examined. The goal of this study was to develop a clinically relevant injectable hydrogel derived from decellularized skeletal muscle extracellular matrix and examine its potential for treating PAD as a stand-alone therapy by studying the material in a rat hindlimb ischemia model. We tested the mitogenic activity of the scaffold's degradation products using an in vitro assay and measured increased proliferation rates of smooth muscle cells and skeletal myoblasts compared to collagen. In a rat hindlimb ischemia model, the femoral artery was ligated and resected, followed by injection of 150 µL of skeletal muscle matrix or collagen 1 week post-injury. We demonstrate that the skeletal muscle matrix increased arteriole and capillary density, as well as recruited more desmin-positive and MyoD-positive cells compared to collagen. Our results indicate that this tissue-specific injectable hydrogel may be a potential therapy for treating ischemia related to PAD, as well as have potential beneficial effects on restoring muscle mass that is typically lost in CLI.
Figures


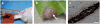





References
-
- Alev C, Ii M, Asahara T. Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxid Redox Signal. 2011;15:949–965. - PubMed
-
- Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. - PubMed
-
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous