Anococcygeal raphe revisited: a histological study using mid-term human fetuses and elderly cadavers
- PMID: 22665356
- PMCID: PMC3381476
- DOI: 10.3349/ymj.2012.53.4.849
Anococcygeal raphe revisited: a histological study using mid-term human fetuses and elderly cadavers
Abstract
Purpose: We recently demonstrated the morphology of the anococcygeal ligament. As the anococcygeal ligament and raphe are often confused, the concept of the anococcygeal raphe needs to be re-examined from the perspective of fetal development, as well as in terms of adult morphology.
Materials and methods: We examined the horizontal sections of 15 fetuses as well as adult histology. From cadavers, we obtained an almost cubic tissue mass containing the dorsal wall of the anorectum, the coccyx and the covering skin. Most sections were stained with hematoxylin and eosin or Masson-trichrome solution.
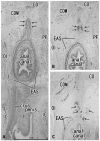
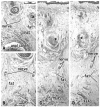
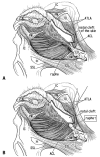
Results: The adult ligament contained both smooth and striated muscle fibers. A similar band-like structure was seen in fetuses, containing: 1) smooth muscle fibers originating from the longitudinal muscle coat of the anal canal and 2) striated muscle fibers from the external anal sphincter (EAS). However, in fetuses, the levator ani muscle did not attach to either the band or the coccyx. Along and around the anococcygeal ligament, we did not find any aponeurotic tissue with transversely oriented fibers connecting bilateral levator ani slings. Instead, in adults, a fibrous tissue mass was located at a gap between bilateral levator ani slings; this site corresponded to the dorsal side of the ligament and the EAS in the immediately deep side of the natal skin cleft.
Conclusion: We hypothesize that a classically described raphe corresponds to the specific subcutaneous tissue on the superficial or dorsal side of the anococcygeal ligament.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



Similar articles
-
The anococcygeal ligaments: Cadaveric study with application to our understanding of incontinence in the elderly.Clin Anat. 2015 Nov;28(8):1039-47. doi: 10.1002/ca.22629. Epub 2015 Oct 13. Clin Anat. 2015. PMID: 26379206
-
Female longitudinal anal muscles or conjoint longitudinal coats extend into the subcutaneous tissue along the vaginal vestibule: a histological study using human fetuses.Yonsei Med J. 2013 May 1;54(3):778-84. doi: 10.3349/ymj.2013.54.3.778. Yonsei Med J. 2013. PMID: 23549829 Free PMC article.
-
Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation).Okajimas Folia Anat Jpn. 2010 Aug;87(2):49-58. doi: 10.2535/ofaj.87.49. Okajimas Folia Anat Jpn. 2010. PMID: 20882767
-
Fascial Organisation and Lymphatic Systems Around the Pelvic Floor: A Literature Review.Anticancer Res. 2021 Oct;41(10):4705-4714. doi: 10.21873/anticanres.15284. Anticancer Res. 2021. PMID: 34593418 Review.
-
Anorectal and pelvic floor anatomy.Best Pract Res Clin Gastroenterol. 2009;23(4):463-75. doi: 10.1016/j.bpg.2009.04.008. Best Pract Res Clin Gastroenterol. 2009. PMID: 19647683 Review.
Cited by
-
A missing distal complex of the external and internal anal sphincters: a macroscopic and histologic study using Japanese and German elderly cadavers.Surg Radiol Anat. 2021 May;43(5):775-784. doi: 10.1007/s00276-020-02606-4. Epub 2020 Nov 2. Surg Radiol Anat. 2021. PMID: 33135107
-
The urethral rhabdosphincter, levator ani muscle, and perineal membrane: a review.Biomed Res Int. 2014;2014:906921. doi: 10.1155/2014/906921. Epub 2014 Apr 27. Biomed Res Int. 2014. PMID: 24877147 Free PMC article. Review.
-
3D Topography of the Young Adult Anal Sphincter Complex Reconstructed from Undeformed Serial Anatomical Sections.PLoS One. 2015 Aug 25;10(8):e0132226. doi: 10.1371/journal.pone.0132226. eCollection 2015. PLoS One. 2015. PMID: 26305117 Free PMC article.
-
Pelvic floor and perineal muscles: a dynamic coordination between skeletal and smooth muscles on pelvic floor stabilization.Anat Sci Int. 2023 Jul;98(3):407-425. doi: 10.1007/s12565-023-00717-7. Epub 2023 Mar 24. Anat Sci Int. 2023. PMID: 36961619 Free PMC article. Review.
-
Dynamic intersection of the longitudinal muscle and external anal sphincter in the layered structure of the anal canal posterior wall.Surg Radiol Anat. 2014 Aug;36(6):551-9. doi: 10.1007/s00276-013-1228-8. Epub 2013 Nov 21. Surg Radiol Anat. 2014. PMID: 24258358
References
-
- Kinugasa Y, Arakawa T, Abe S, Ohtsuka A, Suzuki D, Murakami G, et al. Anatomical reevaluation of the anococcygeal ligament and its surgical relevance. Dis Colon Rectum. 2011;54:232–237. - PubMed
-
- Bogduk N. Issues in anatomy: the external anal sphincter revisited. Aust N Z J Surg. 1996;66:626–629. - PubMed
-
- Borley NR. Anal canal. In: Standring S, editor. Gray's Anatomy. 40 ed. London: Elsevier Churchill Linvingstone; 2008. pp. 1155–1160.
-
- Toldt BvC. Atlas of Human Anatomy for Students and Surgeons. Berlin: Urban & Schwarzenberg; 1903.
-
- Gräfenberg E. Development of the human pelvic musculature. Anat Hefte. 1904;72:429–494.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

