Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages
- PMID: 22665377
- PMCID: PMC3434575
- DOI: 10.1128/IAI.00399-12
Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages
Abstract
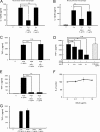
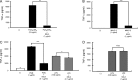
Staphylococcal superantigen-like proteins (SSLs) are a family of exoproteins sharing structural similarity with superantigens, but no superantigenic activity. Corresponding host target proteins or receptors against a portion of SSLs in the family have been identified. In this study, we show that SSL3 specifically binds to Toll-like receptor 2 (TLR2) and inhibits the stimulation of macrophages by TLR2 ligands. An approximately 100-kDa protein was recovered by using recombinant His-tagged SSL3-conjugated Sepharose from the lysate of porcine spleen, and the protein was identified as porcine TLR2 by peptide mass fingerprinting analysis. The SSL3-conjugated Sepharose recovered human and mouse TLR2 but not TLR4 from human neutrophils and mouse macrophage RAW 264.7 cells, as well as a recombinant TLR2 extracellular domain chimera protein. The production levels of interleukin 12 (IL-12) from mouse macrophages treated with heat-killed Staphylococcus aureus and of tumor necrosis factor alpha (TNF-α) from RAW 264.7 cells induced by peptidoglycan or lipopeptide TLR2 ligands were strongly suppressed in the presence of SSL3. The mutation of consensus sialic acid-containing glycan-binding residues in SSL3 did not abrogate the binding ability to TLR2 or inhibitory activity on TLR2, indicating that the interaction of SSL3 with TLR2 was independent of the sialic acid-containing glycan-binding residues. These findings demonstrate that SSL3 is able to bind the extracellular domain of TLR2 and interfere with TLR2 function. The present study provides a novel mechanism of SSL3 in immune evasion of S. aureus via interfering with its recognition by innate immune cells.
Figures


Similar articles
-
Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3.J Mol Med (Berl). 2012 Oct;90(10):1109-20. doi: 10.1007/s00109-012-0926-8. Epub 2012 Jun 20. J Mol Med (Berl). 2012. PMID: 22714643
-
The TLR2 Antagonist Staphylococcal Superantigen-Like Protein 3 Acts as a Virulence Factor to Promote Bacterial Pathogenicity in vivo.J Innate Immun. 2017;9(6):561-573. doi: 10.1159/000479100. Epub 2017 Sep 1. J Innate Immun. 2017. PMID: 28858870
-
Molecular basis determining species specificity for TLR2 inhibition by staphylococcal superantigen-like protein 3 (SSL3).Vet Res. 2018 Nov 28;49(1):115. doi: 10.1186/s13567-018-0609-8. Vet Res. 2018. PMID: 30486901 Free PMC article.
-
Forkhead Box O1 Regulates Macrophage Polarization Following Staphylococcus aureus Infection: Experimental Murine Data and Review of the Literature.Clin Rev Allergy Immunol. 2016 Dec;51(3):353-369. doi: 10.1007/s12016-016-8531-1. Clin Rev Allergy Immunol. 2016. PMID: 26924010 Review.
-
The function of TLR2 during staphylococcal diseases.Front Cell Infect Microbiol. 2013 Jan 4;2:167. doi: 10.3389/fcimb.2012.00167. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316483 Free PMC article. Review.
Cited by
-
Regulation of the Staphylococcal Superantigen-Like Protein 1 Gene of Community-Associated Methicillin-Resistant Staphylococcus aureus in Murine Abscesses.Toxins (Basel). 2019 Jul 4;11(7):391. doi: 10.3390/toxins11070391. Toxins (Basel). 2019. PMID: 31277443 Free PMC article.
-
Humoral immune consequences of Staphylococcus aureus ST239-associated bacteremia.Eur J Clin Microbiol Infect Dis. 2018 Feb;37(2):255-263. doi: 10.1007/s10096-017-3124-3. Epub 2017 Nov 4. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29103153
-
Staphylococcal superantigen-like protein 10 enhances the amyloidogenic biofilm formation in Staphylococcus aureus.BMC Microbiol. 2023 Dec 7;23(1):390. doi: 10.1186/s12866-023-03134-y. BMC Microbiol. 2023. PMID: 38062361 Free PMC article.
-
Categorizing Sequences of Concern by Function To Better Assess Mechanisms of Microbial Pathogenesis.Infect Immun. 2022 May 19;90(5):e0033421. doi: 10.1128/IAI.00334-21. Epub 2021 Nov 15. Infect Immun. 2022. PMID: 34780277 Free PMC article. Review.
-
Cloning, expression, crystallization and preliminary X-ray diffraction studies of staphylococcal superantigen-like protein 1 (SSL1).Acta Crystallogr F Struct Biol Commun. 2014 May;70(Pt 5):600-3. doi: 10.1107/S2053230X14006967. Epub 2014 Apr 15. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24817718 Free PMC article.
References
-
- Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 - PubMed
-
- Baker HM, et al. 2007. Crystal structures of the staphylococcal toxin SSL5 in complex with sialyl Lewis X reveal a conserved binding site that shares common features with viral and bacterial sialic acid binding proteins. J. Mol. Biol. 374:1298–1308 - PubMed
-
- Bestebroer J, et al. 2007. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109:2936–2943 - PubMed
-
- Bestebroer J, et al. 2009. Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood 113:328–337 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

