Brittle culm15 encodes a membrane-associated chitinase-like protein required for cellulose biosynthesis in rice
- PMID: 22665444
- PMCID: PMC3425189
- DOI: 10.1104/pp.112.195529
Brittle culm15 encodes a membrane-associated chitinase-like protein required for cellulose biosynthesis in rice
Abstract
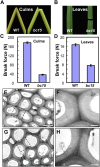
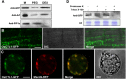
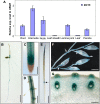
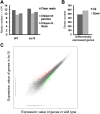
Plant chitinases, a class of glycosyl hydrolases, participate in various aspects of normal plant growth and development, including cell wall metabolism and disease resistance. The rice (Oryza sativa) genome encodes 37 putative chitinases and chitinase-like proteins. However, none of them has been characterized at the genetic level. In this study, we report the isolation of a brittle culm mutant, bc15, and the map-based cloning of the BC15/OsCTL1 (for chitinase-like1) gene affected in the mutant. The gene encodes the rice chitinase-like protein BC15/OsCTL1. Mutation of BC15/OsCTL1 causes reduced cellulose content and mechanical strength without obvious alterations in plant growth. Bioinformatic analyses indicated that BC15/OsCTL1 is a class II chitinase-like protein that is devoid of both an amino-terminal cysteine-rich domain and the chitinase activity motif H-E-T-T but possesses an amino-terminal transmembrane domain. Biochemical assays demonstrated that BC15/OsCTL1 is a Golgi-localized type II membrane protein that lacks classical chitinase activity. Quantitative real-time polymerase chain reaction and β-glucuronidase activity analyses indicated that BC15/OsCTL1 is ubiquitously expressed. Investigation of the global expression profile of wild-type and bc15 plants, using Illumina RNA sequencing, further suggested a possible mechanism by which BC15/OsCTL1 mediates cellulose biosynthesis and cell wall remodeling. Our findings provide genetic evidence of a role for plant chitinases in cellulose biosynthesis in rice, which appears to differ from their roles as revealed by analysis of Arabidopsis (Arabidopsis thaliana).
Figures



Similar articles
-
BC10, a DUF266-containing and Golgi-located type II membrane protein, is required for cell-wall biosynthesis in rice (Oryza sativa L.).Plant J. 2009 Feb;57(3):446-62. doi: 10.1111/j.1365-313X.2008.03703.x. Epub 2008 Oct 2. Plant J. 2009. PMID: 18939965
-
Brittle Culm 15 mutation alters carbohydrate composition, degradation and methanogenesis of rice straw during in vitro ruminal fermentation.Front Plant Sci. 2022 Aug 5;13:975456. doi: 10.3389/fpls.2022.975456. eCollection 2022. Front Plant Sci. 2022. PMID: 35991441 Free PMC article.
-
Members of a new group of chitinase-like genes are expressed preferentially in cotton cells with secondary walls.Plant Mol Biol. 2004 Feb;54(3):353-72. doi: 10.1023/B:PLAN.0000036369.55253.dd. Plant Mol Biol. 2004. PMID: 15284492
-
Structural and functional evolution of chitinase-like proteins from plants.Proteomics. 2015 May;15(10):1693-705. doi: 10.1002/pmic.201400421. Epub 2015 Apr 23. Proteomics. 2015. PMID: 25728311 Review.
-
Chitinases and chitinase-like proteins as biomarkers in neurologic disorders.Neurol Neuroimmunol Neuroinflamm. 2020 Dec 8;8(1):e921. doi: 10.1212/NXI.0000000000000921. Print 2021 Jan. Neurol Neuroimmunol Neuroinflamm. 2020. PMID: 33293459 Free PMC article. Review.
Cited by
-
Combined Transcriptome and Proteome Analysis of Masson Pine (Pinus massoniana Lamb.) Seedling Root in Response to Nitrate and Ammonium Supplementations.Int J Mol Sci. 2020 Oct 13;21(20):7548. doi: 10.3390/ijms21207548. Int J Mol Sci. 2020. PMID: 33066140 Free PMC article.
-
Building an extensible cell wall.Plant Physiol. 2022 Jun 27;189(3):1246-1277. doi: 10.1093/plphys/kiac184. Plant Physiol. 2022. PMID: 35460252 Free PMC article.
-
Mutation of rice bc1 gene affects internode elongation and induces delayed cell wall deposition in developing internodes.Plant Signal Behav. 2020 May 3;15(5):1749786. doi: 10.1080/15592324.2020.1749786. Epub 2020 Apr 16. Plant Signal Behav. 2020. PMID: 32299283 Free PMC article.
-
Identification and Analysis of the Mechanism of Stem Mechanical Strength Enhancement for Maize Inbred Lines QY1.Int J Mol Sci. 2024 Jul 27;25(15):8195. doi: 10.3390/ijms25158195. Int J Mol Sci. 2024. PMID: 39125770 Free PMC article.
-
No Stakes for High Strength Corn.Plant Physiol. 2019 Nov;181(3):845-846. doi: 10.1104/pp.19.01151. Plant Physiol. 2019. PMID: 31685685 Free PMC article. No abstract available.
References
-
- Ancillo G, Witte B, Schmelzer E, Kombrink E. (1999) A distinct member of the basic (class I) chitinase gene family in potato is specifically expressed in epidermal cells. Plant Mol Biol 39: 1137–1151 - PubMed
-
- Audic S, Claverie J-M. (1997) The significance of digital gene expression profiles. Genome Res 7: 986–995 - PubMed
-
- Brogue K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R. (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254: 1194–1197 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

