Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity
- PMID: 22665479
- PMCID: PMC3408201
- DOI: 10.1074/jbc.M112.378323
Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity
Erratum in
- J Biol Chem. 2012 Sep 7;287(37):31456. Bertin, John J [corrected to Bertin, John]
Abstract
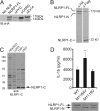
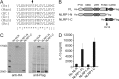
Nucleotide-binding domain leucine-rich repeat proteins (NLRs) play a key role in immunity and disease through their ability to modulate inflammation in response to pathogen-derived and endogenous danger signals. Here, we identify the requirements for activation of NLRP1, an NLR protein associated with a number of human pathologies, including vitiligo, rheumatoid arthritis, and Crohn disease. We demonstrate that NLRP1 activity is dependent upon ASC, which associates with the C-terminal CARD domain of NLRP1. In addition, we show that NLRP1 activity is dependent upon autolytic cleavage at Ser(1213) within the FIIND. Importantly, this post translational event is dependent upon the highly conserved distal residue His(1186). A disease-associated single nucleotide polymorphism near His(1186) and a naturally occurring mRNA splice variant lacking exon 14 differentially affect this autolytic processing and subsequent NLRP1 activity. These results describe key molecular pathways that regulate NLRP1 activity and offer insight on how small sequence variations in NLR genes may influence human disease pathogenesis.
Figures


References
-
- Martinon F., Burns K., Tschopp J. (2002) The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of pro-IL-β. Mol. Cell 10, 417–426 - PubMed
-
- Srinivasula S. M., Poyet J. L., Razmara M., Datta P., Zhang Z., Alnemri E. S. (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277, 21119–21122 - PubMed
-
- Wang L., Manji G. A., Grenier J. M., Al-Garawi A., Merriam S., Lora J. M., Geddes B. J., Briskin M., DiStefano P. S., Bertin J. (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 - PubMed
-
- Geddes B. J., Wang L., Huang W. J., Lavellee M., Manji G. A., Brown M., Jurman M., Cao J., Morgenstern J., Merriam S., Glucksmann M. A., DiStefano P. S., Bertin J. (2001) Human CARD12 is a novel CED4/Apaf-1 family member that induces apoptosis. Biochem. Biophys. Res. Commun. 284, 77–82 - PubMed
-
- Aganna E., Martinon F., Hawkins P. N., Ross J. B., Swan D. C., Booth D. R., Lachmann H. J., Bybee A., Gaudet R., Woo P., Feighery C., Cotter F. E., Thome M., Hitman G. A., Tschopp J., McDermott M. F. (2002) Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

