The protein Zfand5 binds and stabilizes mRNAs with AU-rich elements in their 3'-untranslated regions
- PMID: 22665488
- PMCID: PMC3408148
- DOI: 10.1074/jbc.M112.362020
The protein Zfand5 binds and stabilizes mRNAs with AU-rich elements in their 3'-untranslated regions
Abstract
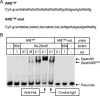
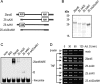
AU-rich elements (AREs) in the 3'-UTR of unstable transcripts play a vital role in the regulation of many inflammatory mediators. To identify novel ARE-dependent gene regulators, we screened a human leukocyte cDNA library for candidates that enhanced the activity of a luciferase reporter bearing the ARE sequence from TNF (ARE(TNF)). Among 171 hits, we focused on Zfand5 (zinc finger, AN1-type domain 5), a 23-kDa protein containing two zinc finger domains. Zfand5 expression was induced in macrophages in response to IFNγ and Toll-like receptor ligands. Knockdown of Zfand5 in macrophages decreased expression of ARE class II transcripts TNF and COX2, whereas overexpression stabilized TNF mRNA by suppressing deadenylation. Zfand5 specifically bound to ARE(TNF) mRNA and competed with tristetraprolin, a protein known to bind and destabilize class II ARE-containing RNAs. Truncation studies indicated that both zinc fingers of Zfand5 contributed to its mRNA-stabilizing function. These findings add Zfand5 to the growing list of RNA-binding proteins and suggest that Zfand5 can enhance ARE-containing mRNA stability by competing with tristetraprolin for mRNA binding.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

