Transcriptional regulation of the protocadherin β cluster during Her-2 protein-induced mammary tumorigenesis results from altered N-glycan branching
- PMID: 22665489
- PMCID: PMC3408139
- DOI: 10.1074/jbc.M112.369355
Transcriptional regulation of the protocadherin β cluster during Her-2 protein-induced mammary tumorigenesis results from altered N-glycan branching
Abstract
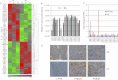
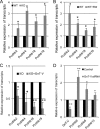
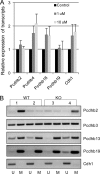
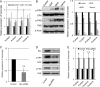
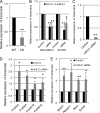
Changes in the levels of N-acetylglucosaminyltransferase V (GnT-V) can alter the function of several types of cell surface receptors and adhesion molecules by causing altered N-linked glycan branching. Using a her-2 mammary tumor mouse model, her-2 receptor signaling was down-regulated by GnT-V knock-out, resulting in a significant delay in the onset of her-2-induced mammary tumors. To identify the genes that contributed to this GnT-V regulation of early events in tumorigenesis, microarray analysis was performed using her-2 induced mammary tumors from wild-type and GnT-V-null mice. We found that 142 genes were aberrantly expressed (>2.0-fold) with 64 genes up-regulated and 78 genes down-regulated after deletion of GnT-V. Among differentially expressed genes, the expression of a subgroup of the cadherin superfamily, the protocadherin β (Pcdhβ) cluster, was up-regulated in GnT-V-null tumors. Altered expression of the Pcdhβ cluster in GnT-V-null tumors was not due to changes in promoter methylation; instead, impaired her-2-mediated signaling pathways were implicated at least in part resulting from reduced microRNA-21 expression. Overexpression of Pcdhβ genes inhibited tumor cell growth, decreased the proportion of tumor-initiating cells, and decreased tumor formation in vivo, demonstrating that expression of the Pcdhβ gene cluster can serve as an inhibitor of the transformed phenotype. Our results suggest the up-regulation of the Pcdhβ gene cluster as a mechanism for reduced her-2-mediated tumorigenesis resulting from GnT-V deletion.
Figures

Similar articles
-
Deletion of mouse embryo fibroblast N-acetylglucosaminyltransferase V stimulates alpha5beta1 integrin expression mediated by the protein kinase C signaling pathway.J Biol Chem. 2005 Mar 4;280(9):8332-42. doi: 10.1074/jbc.M413532200. Epub 2004 Dec 22. J Biol Chem. 2005. PMID: 15615721
-
Specific posttranslational modification regulates early events in mammary carcinoma formation.Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21116-21. doi: 10.1073/pnas.1013405107. Epub 2010 Nov 15. Proc Natl Acad Sci U S A. 2010. PMID: 21078982 Free PMC article.
-
Post-translational glycoprotein modifications regulate colon cancer stem cells and colon adenoma progression in Apc(min/+) mice through altered Wnt receptor signaling.J Biol Chem. 2014 Nov 7;289(45):31534-49. doi: 10.1074/jbc.M114.602680. Epub 2014 Oct 1. J Biol Chem. 2014. PMID: 25274627 Free PMC article.
-
Implication of N-acetylglucosaminyltransferases III and V in cancer: gene regulation and signaling mechanism.Biochim Biophys Acta. 1999 Oct 8;1455(2-3):287-300. doi: 10.1016/s0925-4439(99)00066-6. Biochim Biophys Acta. 1999. PMID: 10571019 Review.
-
True significance of N-acetylglucosaminyltransferases GnT-III, V and α1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer.Mol Aspects Med. 2021 Jun;79:100905. doi: 10.1016/j.mam.2020.100905. Epub 2020 Sep 30. Mol Aspects Med. 2021. PMID: 33010941 Review.
Cited by
-
Epigenetically regulated PCDHB15 impairs aggressiveness of metastatic melanoma cells.Clin Epigenetics. 2022 Nov 28;14(1):156. doi: 10.1186/s13148-022-01364-x. Clin Epigenetics. 2022. PMID: 36443814 Free PMC article.
-
Decreased miR-124-3p promoted breast cancer proliferation and metastasis by targeting MGAT5.Am J Cancer Res. 2019 Mar 1;9(3):585-596. eCollection 2019. Am J Cancer Res. 2019. PMID: 30949412 Free PMC article.
-
Comparison of analytical methods for profiling N- and O-linked glycans from cultured cell lines : HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study.Glycoconj J. 2016 Jun;33(3):405-415. doi: 10.1007/s10719-015-9625-3. Epub 2015 Oct 28. Glycoconj J. 2016. PMID: 26511985 Free PMC article.
-
Protein N-glycosylation in oral cancer: dysregulated cellular networks among DPAGT1, E-cadherin adhesion and canonical Wnt signaling.Glycobiology. 2014 Jul;24(7):579-91. doi: 10.1093/glycob/cwu031. Epub 2014 Apr 17. Glycobiology. 2014. PMID: 24742667 Free PMC article. Review.
-
ISLR interacts with MGAT5 to promote the malignant progression of human gastric cancer AGS cells.Iran J Basic Med Sci. 2023;26(8):960-965. doi: 10.22038/IJBMS.2023.69372.15120. Iran J Basic Med Sci. 2023. PMID: 37427332 Free PMC article.
References
-
- Boscher C., Dennis J. W., Nabi I. R. (2011) Glycosylation, galectins and cellular signaling. Curr. Opin. Cell Biol. 23, 383–392 - PubMed
-
- Granovsky M., Fata J., Pawling J., Muller W. J., Khokha R., Dennis J. W. (2000) Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6, 306–312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

