Ubiquitylation-dependent negative regulation of WASp is essential for actin cytoskeleton dynamics
- PMID: 22665495
- PMCID: PMC3434509
- DOI: 10.1128/MCB.00161-12
Ubiquitylation-dependent negative regulation of WASp is essential for actin cytoskeleton dynamics
Abstract
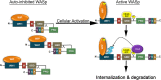
The Wiskott-Aldrich syndrome protein (WASp) is a key regulator of actin dynamics during cell motility and adhesion, and mutations in its gene are responsible for Wiskott-Aldrich syndrome (WAS). Here, we demonstrate that WASp is ubiquitylated following T-cell antigen receptor (TCR) activation. WASp phosphorylation at tyrosine 291 results in recruitment of the E3 ligase Cbl-b, which, together with c-Cbl, carries out WASp ubiquitylation. Lysine residues 76 and 81, located at the WASp WH1 domain, which contains the vast majority of WASp gene mutations, serve as the ubiquitylation sites. Disruption of WASp ubiquitylation causes WASp accumulation and alters actin dynamics and the formation of actin-dependent structures. Our data suggest that regulated degradation of activated WASp might be an efficient strategy by which the duration and localization of actin rearrangement and the intensity of T-cell activation are controlled.
Figures



References
-
- Badour K, Zhang J, Siminovitch KA. 2004. Involvement of the Wiskott-Aldrich syndrome protein and other actin regulatory adaptors in T cell activation. Semin. Immunol. 16:395–407 - PubMed
-
- Badour K, Zhang J, Siminovitch KA. 2003. The Wiskott-Aldrich syndrome protein: forging the link between actin and cell activation. Immunol. Rev. 192:98–112 - PubMed
-
- Barda-Saad M, et al. 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6:80–89 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
