The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection
- PMID: 22665498
- PMCID: PMC3434515
- DOI: 10.1128/MCB.00086-12
The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection
Abstract
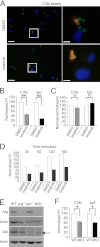
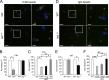
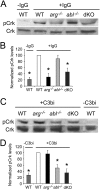
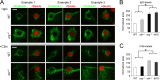
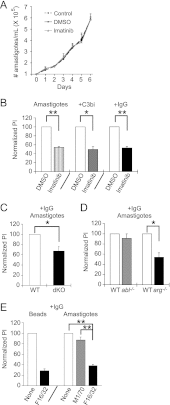
Leishmania, an obligate intracellular parasite, binds several receptors to trigger engulfment by phagocytes, leading to cutaneous or visceral disease. These receptors include complement receptor 3 (CR3), used by promastigotes, and the Fc receptor (FcR), used by amastigotes. The mechanisms mediating uptake are not well understood. Here we show that Abl family kinases mediate both phagocytosis and the uptake of Leishmania amazonensis by macrophages (Ms). Imatinib, an Abl/Arg kinase inhibitor, decreases opsonized polystyrene bead phagocytosis and Leishmania uptake. Interestingly, phagocytosis of IgG-coated beads is decreased in Arg-deficient Ms, while that of C3bi-coated beads is unaffected. Conversely, uptake of C3bi-coated beads is decreased in Abl-deficient Ms, but that of IgG-coated beads is unaffected. Consistent with these results, Abl-deficient Ms are inefficient at C3bi-opsonized promastigote uptake, and Arg-deficient Ms are defective in IgG1-opsonized amastigote uptake. Finally, genetic loss of Abl or Arg reduces infection severity in murine cutaneous leishmaniasis, and imatinib treatment results in smaller lesions with fewer parasites than in controls. Our studies are the first to demonstrate that efficient phagocytosis and maximal Leishmania infection require Abl family kinases. These results highlight Abl family kinase-mediated signaling pathways as potential therapeutic targets for leishmaniasis.
Figures
Similar articles
-
The Src kinases Hck, Fgr and Lyn activate Arg to facilitate IgG-mediated phagocytosis and Leishmania infection.J Cell Sci. 2016 Aug 15;129(16):3130-43. doi: 10.1242/jcs.185595. Epub 2016 Jun 29. J Cell Sci. 2016. PMID: 27358479 Free PMC article.
-
Using machine learning to dissect host kinases required for Leishmania internalization and development.Mol Biochem Parasitol. 2024 Dec;260:111651. doi: 10.1016/j.molbiopara.2024.111651. Epub 2024 Aug 22. Mol Biochem Parasitol. 2024. PMID: 39181505
-
MAPK/ERK activation in macrophages promotes Leishmania internalization and pathogenesis.Microbes Infect. 2024 Jul-Aug;26(5-6):105353. doi: 10.1016/j.micinf.2024.105353. Epub 2024 May 17. Microbes Infect. 2024. PMID: 38763478
-
Unveiling pathways used by Leishmania amazonensis amastigotes to subvert macrophage function.Immunol Rev. 2007 Oct;219:66-74. doi: 10.1111/j.1600-065X.2007.00559.x. Immunol Rev. 2007. PMID: 17850482 Review.
-
Leishmania, macrophages and complement: a tale of subversion and exploitation.Parasitology. 1997;115 Suppl:S9-23. doi: 10.1017/s0031182097001789. Parasitology. 1997. PMID: 9571687 Review.
Cited by
-
Abl family kinases regulate FcγR-mediated phagocytosis in murine macrophages.J Immunol. 2012 Dec 1;189(11):5382-92. doi: 10.4049/jimmunol.1200974. Epub 2012 Oct 24. J Immunol. 2012. PMID: 23100514 Free PMC article.
-
The Src kinases Hck, Fgr and Lyn activate Arg to facilitate IgG-mediated phagocytosis and Leishmania infection.J Cell Sci. 2016 Aug 15;129(16):3130-43. doi: 10.1242/jcs.185595. Epub 2016 Jun 29. J Cell Sci. 2016. PMID: 27358479 Free PMC article.
-
CrkII/Abl phosphorylation cascade is critical for NLRC4 inflammasome activity and is blocked by Pseudomonas aeruginosa ExoT.Nat Commun. 2022 Mar 11;13(1):1295. doi: 10.1038/s41467-022-28967-5. Nat Commun. 2022. PMID: 35277504 Free PMC article.
-
An Antiparasitic Compound from the Medicines for Malaria Venture Pathogen Box Promotes Leishmania Tubulin Polymerization.ACS Infect Dis. 2020 Aug 14;6(8):2057-2072. doi: 10.1021/acsinfecdis.0c00122. Epub 2020 Jul 20. ACS Infect Dis. 2020. PMID: 32686409 Free PMC article.
-
Host-Directed Drug Therapies for Neglected Tropical Diseases Caused by Protozoan Parasites.Front Microbiol. 2018 Nov 30;9:2655. doi: 10.3389/fmicb.2018.02655. eCollection 2018. Front Microbiol. 2018. PMID: 30555425 Free PMC article. Review.
References
-
- Buchdunger E, et al. 2000. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Ther. 295:139–145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
