Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study
- PMID: 22665539
- PMCID: PMC4559602
- DOI: 10.1200/JCO.2011.40.2792
Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study
Abstract
Purpose: We evaluated the feasibility of administering carboplatin as a radiosensitizer during craniospinal radiation therapy (CSRT) to patients with high-risk medulloblastomas (MBs) and supratentorial primitive neuroectodermal tumors, and we report the outcome in the subset with metastatic (M+) MB.
Patients and methods: After surgery, patients received 36 Gy CSRT with boosts to sites of disease. During radiation, patients received 15 to 30 doses of carboplatin (30-45 mg/m(2)/dose), along with vincristine (VCR) once per week for 6 weeks. Patients on regimen A received 6 months of maintenance chemotherapy (MC) with cyclophosphamide and VCR. Once the recommended phase II dose (RP2D) of carboplatin was determined, cisplatin was added to the MC (regimen B).
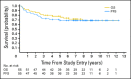
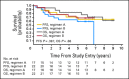
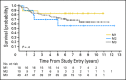
Results: In all, 161 eligible patients (median age, 8.7 years; range, 3.1 to 21.6 years) were enrolled. Myelosuppression was dose limiting and 35 mg/m(2)/dose × 30 was determined to be the RP2D of carboplatin. Twenty-nine (36%) of 81 patients with M+ MB had diffuse anaplasia. Four patients were taken off study within 11 months of completing radiotherapy for presumed metastatic progression and are long-term survivors following palliative chemotherapy. Excluding these four patients, 5-year overall survival ± SE and progression-free survival ± SE for M+ patients treated at the RP2D on regimen A was 82% ± 9% and 71% ± 11% versus 68% ± 10% and 59% ± 10% on regimen B (P = .36). There was no difference in survival by M stage. Anaplasia was a negative predictor of outcome.
Conclusion: The use of carboplatin as a radiosensitizer is a promising strategy for patients with M+ MB. Early progression should be confirmed by biopsy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys. 2000;46:269–279. - PubMed
-
- Bouffet E, Gentet JC, Doz F, et al. Metastatic medulloblastoma: The experience of the French Cooperative M7 Group. Eur J Cancer. 1994;30A:1478–1483. - PubMed
-
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. - PubMed
-
- Taylor RE, Bailey CC, Robinson KJ, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer. 2005;41:727–734. - PubMed
-
- Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

