Naturally acquired and conjugate vaccine-induced antibody to Haemophilus influenzae type b (Hib) polysaccharide in Malian children: serological assessment of the Hib immunization program in Mali
- PMID: 22665612
- PMCID: PMC3366516
- DOI: 10.4269/ajtmh.2012.11-0807
Naturally acquired and conjugate vaccine-induced antibody to Haemophilus influenzae type b (Hib) polysaccharide in Malian children: serological assessment of the Hib immunization program in Mali
Abstract
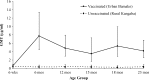
Haemophilus influenzae type b (Hib) conjugate vaccine for infants (6, 10, and 14 weeks of age) was introduced into the Malian Expanded Program on Immunization in July 2005, to diminish invasive Hib disease in young children. Antibodies to Hib capsular polysaccharide (PRP) were measured in infants and toddlers from an area already served by the Hib immunization program (Bamako) and in unimmunized children of the same age in a district (Kangaba) where Hib immunization had not yet begun. Among vaccinated Bamako children 6-23 months of age, 77-93% exhibited PRP titers ≥ 1.0 μg/mL, indicating long-term protection, versus only 10-23% of Kangaba children of that age. High PRP antibody titers in immunized children persisted through 2 years of age. Moreover, ∼50% of Bamako children exhibited anti-PRP titers ≥ 5.0 μg/mL; a level that impedes Hib upper respiratory carriage, and may thereby diminish the Hib transmission to the unimmunized susceptible population (i.e., providing indirect protection).
Figures
Similar articles
-
Haemophilus influenzae Type B conjugate vaccine introduction in Mali: impact on disease burden and serologic correlate of protection.Am J Trop Med Hyg. 2009 Jun;80(6):1033-8. Am J Trop Med Hyg. 2009. PMID: 19478272
-
Lot-to-lot consistency, safety and immunogenicity of 3 lots of Haemophilus influenzae type b conjugate vaccine: results from a phase III randomized, multicenter study in infants.Vaccine. 2017 Jun 16;35(28):3564-3574. doi: 10.1016/j.vaccine.2017.05.018. Epub 2017 May 20. Vaccine. 2017. PMID: 28536030 Clinical Trial.
-
Immunogenicity and safety of two doses of catch-up immunization with Haemophilus influenzae type b conjugate vaccine in Indian children living with HIV.Vaccine. 2016 Apr 27;34(19):2267-74. doi: 10.1016/j.vaccine.2016.03.012. Epub 2016 Mar 14. Vaccine. 2016. PMID: 26988256
-
Circulating innate and adaptive immunity against anti-Haemophilus influenzae type b.Hum Antibodies. 2019;27(3):201-212. doi: 10.3233/HAB-190373. Hum Antibodies. 2019. PMID: 30958343 Review.
-
Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom.Clin Ther. 2012 Feb;34(2):385-99. doi: 10.1016/j.clinthera.2011.11.027. Epub 2012 Jan 12. Clin Ther. 2012. PMID: 22244051 Review.
Cited by
-
Effect of Haemophilus influenzae type b vaccination without a booster dose on invasive H influenzae type b disease, nasopharyngeal carriage, and population immunity in Kilifi, Kenya: a 15-year regional surveillance study.Lancet Glob Health. 2016 Mar;4(3):e185-94. doi: 10.1016/S2214-109X(15)00316-2. Epub 2016 Feb 5. Lancet Glob Health. 2016. PMID: 26853149 Free PMC article.
-
Immune responses to the O-specific polysaccharide antigen in children who received a killed oral cholera vaccine compared to responses following natural cholera infection in Bangladesh.Clin Vaccine Immunol. 2013 Jun;20(6):780-8. doi: 10.1128/CVI.00035-13. Epub 2013 Mar 20. Clin Vaccine Immunol. 2013. PMID: 23515016 Free PMC article.
-
Immunological efficiency of Haemophilus influenzae type b polyribosyl ribitol phosphate combined with detoxified lipooligosaccharide in a rabbit model.Iran J Microbiol. 2025 Feb;17(1):106-112. doi: 10.18502/ijm.v17i1.17807. Iran J Microbiol. 2025. PMID: 40330053 Free PMC article.
-
Immunization Coverage Surveys and Linked Biomarker Serosurveys in Three Regions in Ethiopia.PLoS One. 2016 Mar 2;11(3):e0149970. doi: 10.1371/journal.pone.0149970. eCollection 2016. PLoS One. 2016. PMID: 26934372 Free PMC article. Clinical Trial.
-
Immune responses to vaccine-preventable diseases among toddlers and preschool children after primary immunization and first booster in Northwestern Algiers, Algeria.Heliyon. 2018 Jun 26;4(6):e00664. doi: 10.1016/j.heliyon.2018.e00664. eCollection 2018 Jun. Heliyon. 2018. PMID: 29998194 Free PMC article.
References
-
- World Health Organization WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 2006;81:445–452. - PubMed
-
- Peltola H. Burden of meningitis and other severe bacterial infections of children in Africa: implications for prevention. Clin Infect Dis. 2001;32:64–75. - PubMed
-
- Levine OS, Lagos R, Munoz A, Villaroel J, Alvarez AM, Abrego P, Levine MM. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. - PubMed
-
- Bradshaw MW, Schneerson R, Parke JC, Jr, Robbins JB. Bacterial antigens cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b. Lancet. 1971;1:1095–1096. - PubMed
-
- Robbins JB, Schneerson R, Argaman M, Handzel ZT. Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med. 1973;78:259–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

