Neural reflexes in inflammation and immunity
- PMID: 22665702
- PMCID: PMC3371736
- DOI: 10.1084/jem.20120571
Neural reflexes in inflammation and immunity
Abstract
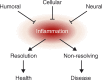
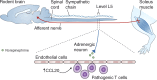
The mammalian immune system and the nervous system coevolved under the influence of infection and sterile injury. Knowledge of homeostatic mechanisms by which the nervous system controls organ function was originally applied to the cardiovascular, gastrointestinal, musculoskeletal, and other body systems. Development of advanced neurophysiological and immunological techniques recently enabled the study of reflex neural circuits that maintain immunological homeostasis, and are essential for health in mammals. Such reflexes are evolutionarily ancient, dating back to invertebrate nematode worms that possess primitive immune and nervous systems. Failure of these reflex mechanisms in mammals contributes to nonresolving inflammation and disease. It is also possible to target these neural pathways using electrical nerve stimulators and pharmacological agents to hasten the resolution of inflammation and provide therapeutic benefit.
Figures


References
-
- Akehi Y., Yoshimatsu H., Kurokawa M., Sakata T., Eto H., Ito S., Ono J. 2001. VLCD-induced weight loss improves heart rate variability in moderately obese Japanese. Exp. Biol. Med. 226:440–445 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

