Photochemically driven redox chemistry induces protocell membrane pearling and division
- PMID: 22665773
- PMCID: PMC3382484
- DOI: 10.1073/pnas.1203212109
Photochemically driven redox chemistry induces protocell membrane pearling and division
Abstract
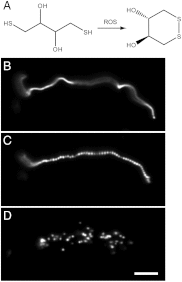
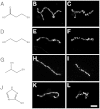
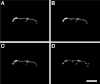
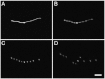
Prior to the evolution of complex biochemical machinery, the growth and division of simple primitive cells (protocells) must have been driven by environmental factors. We have previously demonstrated two pathways for fatty acid vesicle growth in which initially spherical vesicles grow into long filamentous vesicles; division is then mediated by fluid shear forces. Here we describe a different pathway for division that is independent of external mechanical forces. We show that the illumination of filamentous fatty acid vesicles containing either a fluorescent dye in the encapsulated aqueous phase, or hydroxypyrene in the membrane, rapidly induces pearling and subsequent division in the presence of thiols. The mechanism of this photochemically driven pathway most likely involves the generation of reactive oxygen species, which oxidize thiols to disulfide-containing compounds that associate with fatty acid membranes, inducing a change in surface tension and causing pearling and subsequent division. This vesicle division pathway provides an alternative route for the emergence of early self-replicating cell-like structures, particularly in thiol-rich surface environments where UV-absorbing polycyclic aromatic hydrocarbons (PAHs) could have facilitated protocell division. The subsequent evolution of cellular metabolic processes controlling the thiol:disulfide redox state would have enabled autonomous cellular control of the timing of cell division, a major step in the origin of cellular life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409:387–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

