Does size matter in platelet production?
- PMID: 22665937
- PMCID: PMC3429301
- DOI: 10.1182/blood-2012-04-408724
Does size matter in platelet production?
Abstract
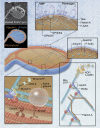
Platelet (PLT) production represents the final stage of megakaryocyte (MK) development. During differentiation, bone marrow MKs extend and release long, branched proPLTs into sinusoidal blood vessels, which undergo repeated abscissions to yield circulating PLTs. Circular-prePLTs are dynamic intermediate structures in this sequence that have the capacity to reversibly convert into barbell-proPLTs and may be related to "young PLTs" and "large PLTs" of both inherited and acquired macrothrombocytopenias. Conversion is regulated by the diameter and thickness of the peripheral microtubule coil, and PLTs are capable of enlarging in culture to generate barbell-proPLTs that divide to yield 2 smaller PLT products. Because PLT number and size are inversely proportional, this raises the question: do macrothrombocytopenias represent a failure in the intermediate stages of PLT production? This review aims to bring together and contextualize our current understanding of terminal PLT production against the backdrop of human macrothrombocytopenias to establish how "large PLTs" observed in both conditions are similar, how they are different, and what they can teach us about PLT formation. A better understanding of the cytoskeletal mechanisms that regulate PLT formation and determine PLT size offers the promise of improved therapies for clinical disorders of PLT production and an important source of PLTs for infusion.
Figures

References
-
- Kessel RG, Kardon RH. Circulating blood, blood vessels, and bone marrow. In: Johnson D, editor. Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy. San Francisco, CA: W.H. Freeman; 1979. pp. 35–50.
-
- Behnke O. An electron microscope study of the rat megacaryocyte: II. Some aspects of platelet release and microtubules. J Ultrastruct Res. 1969;26(1):111–129. - PubMed
-
- Becker RP, De Bruyn PP. The transmural passage of blood cells into myeloid sinusoids and the entry of platelets into the sinusoidal circulation: a scanning electron microscopic investigation. Am J Anat. 1976;145(2):183–205. - PubMed
-
- Larson MK, Watson SP. A product of their environment: do megakaryocytes rely on extracellular cues for proplatelet formation? Platelets. 2006;17(7):435–440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

