A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma
- PMID: 22665938
- PMCID: PMC5162553
- DOI: 10.1182/blood-2012-04-422683
A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma
Abstract
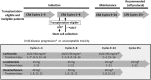
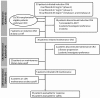
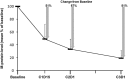
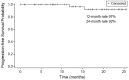
This phase 1/2 study in patients with newly diagnosed multiple myeloma (N = 53) assessed CRd--carfilzomib (20, 27, or 36 mg/m2, days 1, 2, 8, 9, 15, 16 and 1, 2, 15, 16 after cycle 8), lenalidomide (25 mg/d, days 1-21), and weekly dexamethasone (40/20 mg cycles 1-4/5+)--in 28-day cycles. After cycle 4, transplantation-eligible candidates underwent stem cell collection (SCC) then continued CRd with the option of transplantation. The maximum planned dose level (carfilzomib 36 mg/m2) was expanded in phase 2 (n = 36). Thirty-five patients underwent SCC, 7 proceeded to transplantation, and the remainder resumed CRd. Grade 3/4 toxicities included hypophosphatemia (25%), hyperglycemia (23%), anemia (21%), thrombocytopenia (17%), and neutropenia (17%); peripheral neuropathy was limited to grade 1/2 (23%). Most patients did not require dose modifications. After a median of 12 cycles (range, 1-25), 62% (N = 53) achieved at least near-complete response (CR) and 42% stringent CR. Responses were rapid and improved during treatment. In 36 patients completing 8 or more cycles, 78% reached at least near CR and 61% stringent CR. With median follow-up of 13 months (range, 4-25 months), 24-month progression-free survival estimate was 92%. CRd was well tolerated with exceptional response rates. This study is registered at http://www.clinicaltrials.gov as NCT01029054.
Figures
Comment in
-
Treating myeloma: the future is already here!Blood. 2012 Aug 30;120(9):1754-6. doi: 10.1182/blood-2012-06-434571. Blood. 2012. PMID: 22936733 No abstract available.
-
Peripheral neuropathy in multiple myeloma patients receiving lenalidomide, bortezomib, and dexamethasone (RVD) therapy.Blood. 2013 Jan 31;121(5):858. doi: 10.1182/blood-2012-11-465765. Blood. 2013. PMID: 23372151 Free PMC article. No abstract available.
References
-
- Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–4629. - PubMed
-
- Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–436. - PubMed
-
- Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. - PubMed
-
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

