Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report
- PMID: 22666198
- PMCID: PMC3364094
- DOI: 10.1159/000337319
Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report
Abstract
Current therapeutic modalities for ovarian cancer such as chemotherapy, radiotherapy and surgery have been reported to yield only marginal success in improving survival rates of patients and have associated adverse effects. We report here a case of recurrent stage IV ovarian cancer, treated with cell-based autologous immune enhancement therapy (AIET) along with chemotherapy and followed up for 18 months. A 54-year-old female was diagnosed with a recurrence of ovarian carcinoma 1 year after initial surgical removal followed by chemotherapy for stage IIIC ovarian carcinoma. When diagnosed in 2010 with recurrence, she had liver and spleen metastases with a CA-125 level of 243 U/ml and a stage IV clinical status. Six infusions of AIET using autologous in vitro expanded and activated natural killer (NK) cells (CD3-CD56+) and activated T lymphocytes (CD3+CD56+) were administered in combination with 6 cycles of chemotherapy with carboplatin and doxorubicin. Following this treatment, CA-125 decreased to 4.7 U/ml along with regression of the metastatic lesions and an improved quality of life. No adverse reactions were reported after the AIET transfusions. Eighteen months of follow-up revealed a static nonprogressive disease. Combining AIET with chemotherapy and other conventional treatments has been found to be effective in our experience, as reported earlier, even in patients with advanced ovarian cancer, and we recommend this strategy be considered in treating similar cases.
Keywords: Autologous immune enhancement therapy; Natural killer cells (CD3–CD56+); Recurrent ovarian carcinoma; • Activated T lymphocytes (CD3+CD56+).
Figures
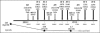

References
-
- Herzog TJ. Recurrent ovarian cancer: how important is it to treat to disease progression? Clin Cancer Res. 2004;10:7439–7449. - PubMed
-
- Modugno F, Ovarian Cancer and High-Risk Women Symposium Presenters Ovarian cancer and high-risk women-implications for prevention, screening, and early detection. Gynecol Oncol. 2003;91:15–31. - PubMed
-
- Lake RA, Robinson BW. Immunotherapy and chemotherapy – a practical partnership. Nat Rev Cancer. 2005;5:397–405. - PubMed
-
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. - PubMed
-
- Kono K, Takahashi A, Ichihara F, Amemiya H, Iizuka H, Fujii H, Sekikawa T, Matsumoto Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res. 2002;8:1767–1771. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

