Autophagy: more than a nonselective pathway
- PMID: 22666256
- PMCID: PMC3362037
- DOI: 10.1155/2012/219625
Autophagy: more than a nonselective pathway
Abstract
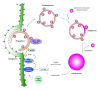
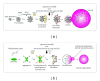
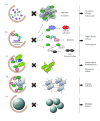
Autophagy is a catabolic pathway conserved among eukaryotes that allows cells to rapidly eliminate large unwanted structures such as aberrant protein aggregates, superfluous or damaged organelles, and invading pathogens. The hallmark of this transport pathway is the sequestration of the cargoes that have to be degraded in the lysosomes by double-membrane vesicles called autophagosomes. The key actors mediating the biogenesis of these carriers are the autophagy-related genes (ATGs). For a long time, it was assumed that autophagy is a bulk process. Recent studies, however, have highlighted the capacity of this pathway to exclusively eliminate specific structures and thus better fulfil the catabolic necessities of the cell. We are just starting to unveil the regulation and mechanism of these selective types of autophagy, but what it is already clearly emerging is that structures targeted to destruction are accurately enwrapped by autophagosomes through the action of specific receptors and adaptors. In this paper, we will briefly discuss the impact that the selective types of autophagy have had on our understanding of autophagy.
Figures
References
-
- Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7(7):673–682. - PubMed
-
- Eskelinen E-L, Reggiori F, Baba M, Kovács AL, Seglen PO. Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy. 2011;7(9):935–956. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

