Rituximab for children with immune thrombocytopenia: a systematic review
- PMID: 22666325
- PMCID: PMC3364261
- DOI: 10.1371/journal.pone.0036698
Rituximab for children with immune thrombocytopenia: a systematic review
Abstract
Background: Rituximab has been widely used off-label as a second line treatment for children with immune thrombocytopenia (ITP). However, its role in the management of pediatric ITP requires clarification. To understand and interpret the available evidence, we conducted a systematic review to assess the efficacy and safety of rituximab for children with ITP.
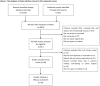
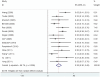
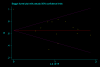
Methodology/principal findings: We searched MEDLINE, EMBASE, Cochrane Library, CBM, CNKI, abstract databases of American Society of Hematology, American Society of Clinical Oncology and Pediatric Academic Society. Clinical studies published in full text or abstract only in any language that met predefined inclusion criteria were eligible. Efficacy analysis was restricted to studies enrolling 5 or more patients. Safety was evaluated from all studies that reported data of toxicity. 14 studies (323 patients) were included for efficacy assessment in children with primary ITP. The pooled complete response (platelet count ≥ 100 × 10(9)/L) and response (platelet count ≥ 30 × 10(9)/L) rate after rituximab treatment were 39% (95% CI, 30% to 49%) and 68% (95%CI, 58% to 77%), respectively, with median response duration of 12.8 month. 4 studies (29 patients) were included for efficacy assessment in children with secondary ITP. 11 (64.7%) of 17 patients associated with Evans syndrome achieved response. All 6 patients with systemic lupus erythematosus associated ITP and all 6 patients with autoimmune lymphoproliferative syndrome associated ITP achieved response. 91 patients experienced 108 adverse events associated with rituximab, among that, 91 (84.3%) were mild to moderate, and no death was reported.
Conclusions/significance: Randomized controlled studies on effect of rituximab for children with ITP are urgently needed, although a series of uncontrolled studies found that rituximab resulted in a good platelet count response both in children with primary and children secondary ITP. Most adverse events associated with rituximab were mild to moderate, and no death was reported.
Conflict of interest statement
Figures

References
-
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, et al. Standardization of terminology, definitions and outcome criteria in an international working group immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. - PubMed
-
- Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, et al. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85:174–180. - PubMed
-
- Kühne T, Buchanan GR, Zimmerman S, Michaels LA, Kohan R, et al. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143:605–608. - PubMed
-
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. - PubMed
-
- Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

