Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5
- PMID: 22666346
- PMCID: PMC3364248
- DOI: 10.1371/journal.pone.0037222
Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5
Erratum in
- PLoS One. 2012;7(10). doi:10.1371/annotation/f7203563-87dc-4d11-a1b7-958f81cf743a
Abstract
Background: Dihydroartemisinin (DHA), a semi-synthetic derivative of artemisinin, has recently shown antitumor activity in various cancer cells. Apo2 ligand or tumor necrosis factor-related apoptosis-inducing ligand (Apo2L/TRAIL) is regarded as a promising anticancer agent, but chemoresistance affects its efficacy as a treatment strategy. Apoptosis induced by the combination of DHA and Apo2L/TRAIL has not been well documented, and the mechanisms involved remain unclear.
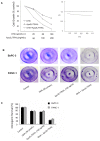
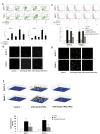
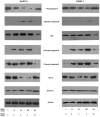
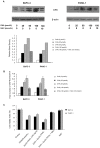
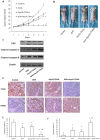
Methodology/principal findings: Here, we report that DHA enhances the efficacy of Apo2L/TRAIL for the treatment of pancreatic cancer. We found that combined therapy using DHA and Apo2L/TRAIL significantly enhanced apoptosis in BxPC-3 and PANC-1 cells compared with single-agent treatment in vitro. The effect of DHA was mediated through the generation of reactive oxygen species, the induction of death receptor 5 (DR5) and the modulation of apoptosis-related proteins. However, N-acetyl cysteine significantly reduced the enhanced apoptosis observed with the combination of DHA and Apo2L/TRAIL. In addition, knockdown of DR5 by small interfering RNA also significantly reduced the amount of apoptosis induced by DHA and Apo2L/TRAIL.
Conclusions/significance: These results suggest that DHA enhances Apo2L/TRAIL-mediated apoptosis in human pancreatic cancer cells through reactive oxygen species-mediated up-regulation of DR5.
Conflict of interest statement
Figures
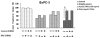
References
-
- Raraty MG, Magee CJ, Ghaneh P, Neoptolemos JP. New techniques and agents in the adjuvant therapy of pancreatic cancer. Acta Oncol. 2002;41:582–595. - PubMed
-
- Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: Challenge of the facts. World J Surg. 2003;27:1075–1084. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–1660. - PubMed
-
- O'Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem. 2004;47:2945–2964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

