Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo
- PMID: 22666347
- PMCID: PMC3364240
- DOI: 10.1371/journal.pone.0037242
Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo
Abstract
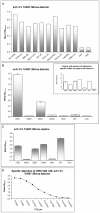
Venezuelan equine encephalitis virus (VEEV) belongs to the Alphavirus genus and several species of this family are pathogenic to humans. The viruses are classified as potential agents of biological warfare and terrorism and sensitive detection as well as effective prophylaxis and antiviral therapies are required.In this work, we describe the isolation of the anti-VEEV single chain Fragment variable (scFv), ToR67-3B4, from a non-human primate (NHP) antibody gene library. We report its recloning into the bivalent scFv-Fc format and further immunological and biochemical characterisation.The scFv-Fc ToR67-3B4 recognised viable as well as formalin and ß-propionolactone (ß-Pl) inactivated virus particles and could be applied for immunoblot analysis of VEEV proteins and immuno-histochemistry of VEEV infected cells. It detected specifically the viral E1 envelope protein of VEEV but did not react with reduced viral glycoprotein preparations suggesting that recognition depends upon conformational epitopes. The recombinant antibody was able to detect multiple VEEV subtypes and displayed only marginal cross-reactivity to other Alphavirus species except for EEEV. In addition, the scFv-Fc fusion described here might be of therapeutic use since it successfully inactivated VEEV in a murine disease model. When the recombinant antibody was administered 6 hours post challenge, 80% to 100% of mice survived lethal VEEV IA/B or IE infection. Forty to sixty percent of mice survived when scFv-Fc ToR67-3B4 was applied 6 hours post challenge with VEEV subtypes II and former IIIA. In combination with E2-neutralising antibodies the NHP antibody isolated here could significantly improve passive protection as well as generic therapy of VEE.
Conflict of interest statement
Figures


References
-
- Kubes V, Ríos FA. The causative agent of infectious Equine Encephalomyelitis in Venezuela. Science. 1939;90:20–21. - PubMed
-
- Reed DS, Lind CM, Sullivan LJ, Pratt WD, Parker MD. Aerosol Infection of Cynomolgus Macaques with Enzootic Strains of Venezuelan Equine Encephalitis Viruses. J Infect Dis. 2004;189(6):1013–1017. - PubMed
-
- Smith JF, Davis K, Hart MK, Ludwig GV, McClain DJ, et al. Textbook of Military Medicine; Medical Aspects of Chemical and Biological Warfare. Washington DC: Office of surgeon general; 1997. Viral encephali-tides. pp. 561–589.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

