Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma
- PMID: 22666367
- PMCID: PMC3364283
- DOI: 10.1371/journal.pone.0037563
Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma
Abstract
Background: Sorafenib induces frequent dose limiting toxicities (DLT) in patients with advanced hepatocellular carcinoma (HCC). Sarcopenia has been associated with poor performance status and shortened survival in cancer patients.
Patients and methods: The characteristics of Child Pugh A cirrhotic patients with HCC receiving sorafenib in our institution were retrospectively analyzed. Sorafenib plasma concentrations were determined at each visit. Toxicities were recorded during the first month of treatment, and sarcopenia was determined from baseline CT-scans.
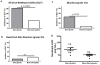
Results: Forty patients (30 males) were included. Eleven (27.5%) were sarcopenic. Eighteen patients (45%) experienced a DLT during the first month of treatment. Sarcopenic patients experienced significantly more DLTs than non-sarcopenic patients did (82% versus 31%, p = 0.005). Grade 3 diarrhea was significantly more frequent in sarcopenic patients than in non-sarcopenic patients (45.5% versus 6.9%, p = 0.01), but not grade 3 hand foot syndrome reaction (9% versus 17.2%, p = 1). On day 28, median sorafenib AUC (n = 17) was significantly higher in sarcopenic patients (102.4 mg/l.h versus 53.7 mg/l.h, p = 0.013).
Conclusions: Among cirrhotic Child Pugh A patients with advanced HCC, sarcopenia predicts sorafenib exposure and the occurrence of DLT within the first month of treatment.
Conflict of interest statement
Figures


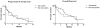
References
-
- Jemal A, Bray F Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
-
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

