Label-free enrichment of functional cardiomyocytes using microfluidic deterministic lateral flow displacement
- PMID: 22666372
- PMCID: PMC3362623
- DOI: 10.1371/journal.pone.0037619
Label-free enrichment of functional cardiomyocytes using microfluidic deterministic lateral flow displacement
Abstract
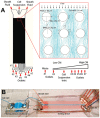
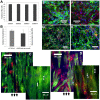
Progress in cardiac cell replacement therapies and tissue engineering critically depends on our ability to isolate functional cardiomyocytes (CMs) from heterogeneous cell mixtures. Label-free enrichment of cardiomyocytes is desirable for future clinical application of cell based products. Taking advantage of the physical properties of CMs, a microfluidic system was designed to separate CMs from neonatal rat heart tissue digest based on size using the principles of deterministic lateral displacement (DLD). For the first time, we demonstrate enrichment of functional CMs up to 91 ± 2.4% directly from the digested heart tissue without any pre-treatment or labeling. Enriched cardiomyocytes remained viable after sorting and formed contractile cardiac patches in 3-dimensional culture. The broad significance of this work lies in demonstrating functional cell enrichment from the primary tissue digest leading directly to the creation of the engineered tissue.
Conflict of interest statement
Figures

Similar articles
-
Deterministic lateral displacement as a means to enrich large cells for tissue engineering.Anal Chem. 2009 Nov 1;81(21):9178-82. doi: 10.1021/ac9018395. Anal Chem. 2009. PMID: 19810716
-
Sorting differentiated mammalian cells using deterministic lateral displacement microfluidic devices.Anal Sci. 2024 Oct;40(10):1801-1807. doi: 10.1007/s44211-024-00634-3. Epub 2024 Jul 26. Anal Sci. 2024. PMID: 39060754
-
Label-Free Multitarget Separation of Particles and Cells under Flow Using Acoustic, Electrophoretic, and Hydrodynamic Forces.Anal Chem. 2021 Jun 1;93(21):7635-7646. doi: 10.1021/acs.analchem.1c00312. Epub 2021 May 20. Anal Chem. 2021. PMID: 34014074
-
Generation and purification of human stem cell-derived cardiomyocytes.Differentiation. 2016 Apr-Jun;91(4-5):126-38. doi: 10.1016/j.diff.2016.01.001. Epub 2016 Feb 22. Differentiation. 2016. PMID: 26915912 Review.
-
Large-Volume Microfluidic Cell Sorting for Biomedical Applications.Annu Rev Biomed Eng. 2015;17:1-34. doi: 10.1146/annurev-bioeng-071114-040818. Epub 2015 Jul 16. Annu Rev Biomed Eng. 2015. PMID: 26194427 Review.
Cited by
-
Microfluidic platforms for monitoring cardiomyocyte electromechanical activity.Microsyst Nanoeng. 2025 Jan 9;11(1):4. doi: 10.1038/s41378-024-00751-z. Microsyst Nanoeng. 2025. PMID: 39788940 Free PMC article. Review.
-
Lab-on-a-Chip Technologies for the Single Cell Level: Separation, Analysis, and Diagnostics.Micromachines (Basel). 2020 Apr 29;11(5):468. doi: 10.3390/mi11050468. Micromachines (Basel). 2020. PMID: 32365567 Free PMC article. Review.
-
Fundamentals and application of magnetic particles in cell isolation and enrichment: a review.Rep Prog Phys. 2015 Jan;78(1):016601. doi: 10.1088/0034-4885/78/1/016601. Epub 2014 Dec 4. Rep Prog Phys. 2015. PMID: 25471081 Free PMC article. Review.
-
Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells.Theranostics. 2017 May 17;7(7):2067-2077. doi: 10.7150/thno.19427. eCollection 2017. Theranostics. 2017. PMID: 28638487 Free PMC article. Review.
-
Charge-Based Separation of Micro- and Nanoparticles.Micromachines (Basel). 2020 Nov 18;11(11):1014. doi: 10.3390/mi11111014. Micromachines (Basel). 2020. PMID: 33218201 Free PMC article.
References
-
- Rosamond W, Flegal K, Furie K, Go A, et al. Writing Group Members. Heart Disease and Stroke Statistics–2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. - PubMed
-
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. - PubMed
-
- Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circulation Research. 1998;83:15–26. - PubMed
-
- Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174–3183. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

