Effect of wall compliance and permeability on blood-flow rate in counter-current microvessels formed from anastomosis during tumor-induced angiogenesis
- PMID: 22667678
- PMCID: PMC3705849
- DOI: 10.1115/1.4006338
Effect of wall compliance and permeability on blood-flow rate in counter-current microvessels formed from anastomosis during tumor-induced angiogenesis
Abstract
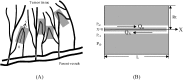
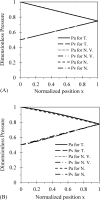
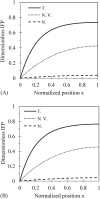
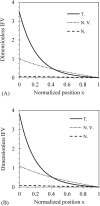
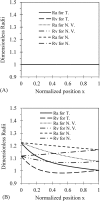
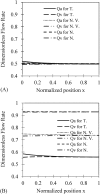
Tumor blood-flow is inhomogeneous because of heterogeneity in tumor vasculature, vessel-wall leakiness, and compliance. Experimental studies have shown that normalization of tumor vasculature by antiangiogenic therapy can improve tumor microcirculation and enhance the delivery of therapeutic agents to tumors. To elucidate the quantitative relationship between the vessel-wall compliance and permeability and the blood-flow rate in the microvessels of the tumor tissue, the tumor tissue with the normalized vasculature, and the normal tissue, we developed a transport model to simultaneously predict the interstitial fluid pressure (IFP), interstitial fluid velocity (IFV) and the blood-flow rate in a counter-current microvessel loop, which occurs from anastomosis in tumor-induced angiogenesis during tumor growth. Our model predicts that although the vessel-wall leakiness greatly affects the IFP and IFV, it has a negligible effect on the intravascular driving force (pressure gradient) for both rigid and compliant vessels, and thus a negligible effect on the blood-flow rate if the vessel wall is rigid. In contrast, the wall compliance contributes moderately to the IFP and IFV, but significantly to the vessel radius and to the blood-flow rate. However, the combined effects of vessel leakiness and compliance can increase IFP, which leads to a partial collapse in the blood vessels and an increase in the flow resistance. Furthermore, our model predictions speculate a new approach for enhancing drug delivery to tumor by modulating the vessel-wall compliance in addition to reducing the vessel-wall leakiness and normalizing the vessel density.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

