Blood-nanoparticle interactions and in vivo biodistribution: impact of surface PEG and ligand properties
- PMID: 22668197
- PMCID: PMC3524348
- DOI: 10.1021/mp200626j
Blood-nanoparticle interactions and in vivo biodistribution: impact of surface PEG and ligand properties
Abstract
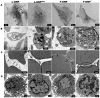
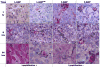
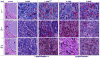
Theranostic nanoparticles (NPs) cannot reach their target tissue without first passing through blood; however, the influence of blood protein and blood cell interactions on NP biodistribution are not well understood. The current work shows that 30 nm PEGylated gold NPs (GNPs) interact not only with blood proteins as thought before but also with blood cells (especially platelets and monocytes) in vivo and that longer blood circulation correlates strongly with tumor uptake. Further, GNP surface properties such as negative charge or lyophilization had either a minimal (i.e., charge) or 15-fold increase (i.e., fresh vs lyophilized) in blood retention times and tumor uptake. Tumor accumulation was increased over 10-fold by use of a bioactive ligand (i.e., TNF) on the lyophilized GNP surface. Resident macrophages were primarily responsible for the bulk of GNP uptake in liver while spleen uptake was highly surface property dependent and appears to involve macrophages and cellular interaction between the red and white pulp. This study shows that the PEG layer and ligand on the surface of the NP are critical to blood interactions and eventual tumor and RES organ biodistribution in vivo.
Figures


Similar articles
-
Integration of Peptides for Enhanced Uptake of PEGylayed Gold Nanoparticles.J Nanosci Nanotechnol. 2015 Mar;15(3):2125-31. doi: 10.1166/jnn.2015.10321. J Nanosci Nanotechnol. 2015. PMID: 26413630
-
Radiosensitization of ultrasmall GNP-PEG-cRGDfK in ALTS1C1 exposed to therapeutic protons and kilovoltage and megavoltage photons.Int J Radiat Biol. 2018 Feb;94(2):124-136. doi: 10.1080/09553002.2018.1407462. Epub 2018 Jan 8. Int J Radiat Biol. 2018. PMID: 29172866
-
Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages.Eur J Pharm Biopharm. 2011 Apr;77(3):417-23. doi: 10.1016/j.ejpb.2010.11.010. Epub 2010 Nov 18. Eur J Pharm Biopharm. 2011. PMID: 21093587 Free PMC article.
-
Advances in biodistribution of gold nanoparticles: the influence of size, surface charge, and route of administration.Biomed Mater. 2024 Jun 21;19(4). doi: 10.1088/1748-605X/ad5484. Biomed Mater. 2024. PMID: 38838693 Review.
-
Polymer Conjugated Gold Nanoparticles in Biomedical Applications.Curr Med Chem. 2018;25(12):1433-1445. doi: 10.2174/0929867324666170116123633. Curr Med Chem. 2018. PMID: 28093984 Review.
Cited by
-
Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics.Sci Rep. 2013;3:1293. doi: 10.1038/srep01293. Sci Rep. 2013. PMID: 23443065 Free PMC article.
-
Neurotheranostics as personalized medicines.Adv Drug Deliv Rev. 2019 Aug;148:252-289. doi: 10.1016/j.addr.2018.10.011. Epub 2018 Oct 26. Adv Drug Deliv Rev. 2019. PMID: 30421721 Free PMC article. Review.
-
Targeted Nanoparticle Binding to Hydroxyapatite in a High Serum Environment for Early Detection of Heart Disease.ACS Appl Nano Mater. 2018 Sep 28;1(9):4927-4939. doi: 10.1021/acsanm.8b01099. Epub 2018 Aug 21. ACS Appl Nano Mater. 2018. PMID: 31867573 Free PMC article.
-
Exploring and Analyzing the Systemic Delivery Barriers for Nanoparticles.Adv Funct Mater. 2024 Feb 19;34(8):2308446. doi: 10.1002/adfm.202308446. Epub 2023 Nov 20. Adv Funct Mater. 2024. PMID: 38828467 Free PMC article.
-
Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes.Pharmaceutics. 2019 Jul 11;11(7):327. doi: 10.3390/pharmaceutics11070327. Pharmaceutics. 2019. PMID: 31336703 Free PMC article. Review.
References
-
- Kim BYS, Rutka JT, Chan WCW. Nanomedicine. New England Journal of Medicine. 2010;363(25):2434–2443. - PubMed
-
- Cytimmune. 2009 http://www.cytimmune.com/go.cfm?do=Page.View&pid=26.
-
- Nanospectra. 2009 http://www.nanospectra.com/technology/aurolasetherapy.html.
-
- Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

