CXCR4 gene transfer prevents pressure overload induced heart failure
- PMID: 22668785
- PMCID: PMC3409693
- DOI: 10.1016/j.yjmcc.2012.05.016
CXCR4 gene transfer prevents pressure overload induced heart failure
Abstract
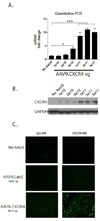
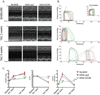
Stem cell and gene therapies are being pursued as strategies for repairing damaged cardiac tissue following myocardial infarction in an attempt to prevent heart failure. The chemokine receptor-4 (CXCR4) and its ligand, CXCL12, play a critical role in stem cell recruitment post-acute myocardial infarction. Whereas progenitor cell migration via the CXCL12/CXCR4 axis is well characterized, little is known about the molecular mechanisms of CXCR4 mediated modulation of cardiac hypertrophy and failure. We used gene therapy to test the effects of CXCR4 gene delivery on adverse ventricular remodeling due to pressure overload. We assessed the effect of cardiac overexpression of CXCR4 during trans-aortic constriction (TAC) using a cardiotropic adeno-associated viral vector (AAV9) carrying the CXCR4 gene. Cardiac overexpression of CXCR4 in mice with pressure overload prevented ventricular remodeling, preserved capillary density and maintained function as determined by echocardiography and in vivo hemodynamics. In isolated adult rat cardiac myocytes, CXCL12 treatment prevented isoproterenol induced hypertrophy and interrupted the calcineurin/NFAT pathway. Finally, a complex involving the L-type calcium channel, β2-adrenoceptor, and CXCR4 (Cav1.2/β2AR/CXCR4) was identified in healthy cardiac myocytes and was shown to dissociate as a consequence of heart failure. CXCR4 administered to the heart via gene transfer prevents pressure overload induced heart failure. The identification of CXCR4 participation in a Cav1.2-β2AR regulatory complex provides further insight into the mechanism by which CXCR4 modulates calcium homeostasis and chronic pressure overload responses in the cardiac myocyte. Together these results suggest that AAV9.CXCR4 gene therapy is a potential therapeutic approach for congestive heart failure.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Deletion of CXCR4 in cardiomyocytes exacerbates cardiac dysfunction following isoproterenol administration.Gene Ther. 2014 May;21(5):496-506. doi: 10.1038/gt.2014.23. Epub 2014 Mar 20. Gene Ther. 2014. PMID: 24646609 Free PMC article.
-
CXCR4 Cardiac Specific Knockout Mice Develop a Progressive Cardiomyopathy.Int J Mol Sci. 2019 May 8;20(9):2267. doi: 10.3390/ijms20092267. Int J Mol Sci. 2019. PMID: 31071921 Free PMC article.
-
CXCR4 modulates contractility in adult cardiac myocytes.J Mol Cell Cardiol. 2006 Nov;41(5):834-44. doi: 10.1016/j.yjmcc.2006.08.008. Epub 2006 Sep 28. J Mol Cell Cardiol. 2006. PMID: 17010372 Free PMC article.
-
A gene therapeutic approach to inhibit calcium and integrin binding protein 1 ameliorates maladaptive remodelling in pressure overload.Cardiovasc Res. 2019 Jan 1;115(1):71-82. doi: 10.1093/cvr/cvy154. Cardiovasc Res. 2019. PMID: 29931050
-
Epoxyeicosatrienoic acid prevents maladaptive remodeling in pressure overload by targeting calcineurin/NFAT and Smad-7.Exp Cell Res. 2020 Jan 1;386(1):111716. doi: 10.1016/j.yexcr.2019.111716. Epub 2019 Nov 14. Exp Cell Res. 2020. PMID: 31734152
Cited by
-
CXCL12 in Patients with Chronic Kidney Disease and Healthy Controls: Relationships to Ambulatory 24-Hour Blood Pressure and Echocardiographic Measures.Cardiorenal Med. 2018;8(3):249-258. doi: 10.1159/000490396. Epub 2018 Jul 18. Cardiorenal Med. 2018. PMID: 30021207 Free PMC article.
-
Bone marrow SSEA1+ cells support the myocardium in cardiac pressure overload.PLoS One. 2013 Jul 9;8(7):e68528. doi: 10.1371/journal.pone.0068528. Print 2013. PLoS One. 2013. PMID: 23874657 Free PMC article.
-
The vestigial enzyme D-dopachrome tautomerase protects the heart against ischemic injury.J Clin Invest. 2014 Aug;124(8):3540-50. doi: 10.1172/JCI73061. Epub 2014 Jul 1. J Clin Invest. 2014. PMID: 24983315 Free PMC article.
-
CXCR4 and CXCR7 play distinct roles in cardiac lineage specification and pharmacologic β-adrenergic response.Stem Cell Res. 2017 Aug;23:77-86. doi: 10.1016/j.scr.2017.06.015. Epub 2017 Jul 8. Stem Cell Res. 2017. PMID: 28711757 Free PMC article.
-
Deletion of CXCR4 in cardiomyocytes exacerbates cardiac dysfunction following isoproterenol administration.Gene Ther. 2014 May;21(5):496-506. doi: 10.1038/gt.2014.23. Epub 2014 Mar 20. Gene Ther. 2014. PMID: 24646609 Free PMC article.
References
-
- Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2010;129(1):97–108. - PubMed
-
- Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55(5):496–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

