Population pharmacokinetics modeling of levetiracetam in Chinese children with epilepsy
- PMID: 22669118
- PMCID: PMC4010372
- DOI: 10.1038/aps.2012.57
Population pharmacokinetics modeling of levetiracetam in Chinese children with epilepsy
Abstract
Aim: To establish a population pharmacokinetics (PPK) model of levetiracetam in Chinese children with epilepsy.
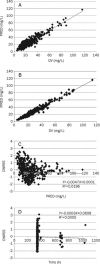
Methods: A total of 418 samples from 361 epileptic children in Peking University First Hospital were analyzed. These patients were divided into two groups: the PPK model group (n=311) and the PPK validation group (n=50). Levetiracetam concentrations were determined by HPLC. The PPK model of levetiracetam was established using NONMEM, according to a one-compartment model with first-order absorption and elimination. To validate the model, the mean prediction error (MPE), mean squared prediction error (MSPE), root mean-squared prediction error (RMSPE), weight residues (WRES), and the 95% confidence intervals (95% CI) were calculated.
Results: A regression equation of the basic model of levetiracetam was obtained, with clearance (CL/F)=0.988 L/h, volume of distribution (V/F)=12.3 L, and K(a)=1.95 h(-1). The final model was as follows: K(a)=1.56 h(-1), V/F=12.1 (L), CL/F=1.04×(WEIG/25)(0.583) (L/h). For the basic model, the MPE, MSPE, RMSPE, WRES, and the 95%CI were 9.834 (-0.587-197.720), 50.919 (0.012-1286.429), 1.680 (0.021-34.184), and 0.0621 (-1.100-1.980). For the final model, the MPE, MSPE, RMSPE, WRES, and the 95% CI were 0.199 (-0.369-0.563), 0.002082 (0.00001-0.01054), 0.0293 (0.001-0.110), and 0.153 (-0.030-1.950).
Conclusion: A one-compartment model with first-order absorption adequately described the levetiracetam concentrations. Body weight was identified as a significant covariate for levetiracetam clearance in this study. This model will be valuable to facilitate individualized dosage regimens.
Figures


Similar articles
-
Population pharmacokinetics of valproate in Chinese children with epilepsy.Acta Pharmacol Sin. 2007 Oct;28(10):1677-84. doi: 10.1111/j.1745-7254.2007.00704.x. Acta Pharmacol Sin. 2007. PMID: 17883957
-
[Population pharmacokinetics of lamotrigine in Chinese children with epilepsy].Zhongguo Dang Dai Er Ke Za Zhi. 2008 Apr;10(2):105-9. Zhongguo Dang Dai Er Ke Za Zhi. 2008. PMID: 18433522 Chinese.
-
Population pharmacokinetic models of lamotrigine in different age groups of Chinese children with epilepsy.Eur J Clin Pharmacol. 2017 Apr;73(4):445-453. doi: 10.1007/s00228-016-2190-2. Epub 2017 Jan 7. Eur J Clin Pharmacol. 2017. PMID: 28064355
-
The pharmacokinetic characteristics of levetiracetam.Methods Find Exp Clin Pharmacol. 2003 Mar;25(2):123-9. doi: 10.1358/mf.2003.25.2.723686. Methods Find Exp Clin Pharmacol. 2003. PMID: 12731458 Review.
-
Pharmacokinetic profile of levetiracetam: toward ideal characteristics.Pharmacol Ther. 2000 Feb;85(2):77-85. doi: 10.1016/s0163-7258(99)00052-2. Pharmacol Ther. 2000. PMID: 10722121 Review.
Cited by
-
Experience and pharmacokinetics of Levetiracetam in Korean neonates with neonatal seizures.Korean J Pediatr. 2017 Feb;60(2):50-54. doi: 10.3345/kjp.2017.60.2.50. Epub 2017 Feb 27. Korean J Pediatr. 2017. PMID: 28289434 Free PMC article.
-
Pharmacokinetics and Pharmacodynamics of Levetiracetam in Neonatal Seizures: What We Still Need to Know.J Pediatr Pharmacol Ther. 2025 Apr;30(2):170-181. doi: 10.5863/1551-6776-30.2.170. Epub 2025 Apr 14. J Pediatr Pharmacol Ther. 2025. PMID: 40717753 Free PMC article.
-
Population pharmacokinetics of levetiracetam in Chinese adult epilepsy patients with varying renal function: exposure simulation and individualized dosing adjustments.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):9267-9278. doi: 10.1007/s00210-025-03816-6. Epub 2025 Feb 10. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39928152
-
Population Pharmacokinetics of Levetiracetam: A Systematic Review.Clin Pharmacokinet. 2021 Mar;60(3):305-318. doi: 10.1007/s40262-020-00963-2. Epub 2021 Jan 15. Clin Pharmacokinet. 2021. PMID: 33447943
-
Levetiracetam Clinical Pharmacokinetic Monitoring in Pediatric Patients with Epilepsy.Clin Pharmacokinet. 2017 Nov;56(11):1267-1285. doi: 10.1007/s40262-017-0537-1. Clin Pharmacokinet. 2017. PMID: 28353057 Review.
References
-
- Stefan H, Feuerstein TJ. Novel anticonvulsant drugs. Pharmacol Ther. 2007;113:165–83. - PubMed
-
- Shorvon SD, Lowenthal A, Janz D. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia. 2000;41:1179–86. - PubMed
-
- Hwang H, Kim KJ. New antiepileptic drugs in pediatric epilepsy. Brain Dec. 2008;30:549–55. - PubMed
-
- Stockist A, Lu S, Tonner F. Clinical pharmacology of levetiracetam for the treatment of epilepsy. Expert Rev Pharmacol. 2009;2:339–50. - PubMed
-
- Zhou B, Zhang Q, Tian L, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav. 2008;12:305–10. - PubMed

