Association between vancomycin-resistant Enterococci bacteremia and ceftriaxone usage
- PMID: 22669234
- PMCID: PMC3879097
- DOI: 10.1086/666331
Association between vancomycin-resistant Enterococci bacteremia and ceftriaxone usage
Abstract
Objective: Vancomycin-resistant enterococci (VRE) have become a public health concern with implications for patient mortality and costs. Hospital antibiotic usage may impact VRE incidence, but the relationship is poorly understood. Animal investigations suggest that ceftriaxone may be associated with VRE proliferation. We measured antimicrobial usage and VRE bloodstream infection (VRE-BSI) incidence to test our hypothesis that increased ceftriaxone usage would be associated with a higher incidence of VRE-BSI.
Design: Retrospective cohort study.
Setting: University of Alabama at Birmingham Medical Center, a 900-bed urban tertiary care hospital.
Participants: All patients admitted during the study period contributed data.
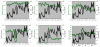
Methods: We conducted a retrospective analysis of antimicrobial usage and VRE-BSI from 2005 to 2008 (43 months). Antimicrobial usage was quantified as days of therapy (DOTs) per 1,000 patient-days. VRE-BSI incidence was calculated as cases per 1,000 patient-days. Negative binomial regression with adjustment for correlation between consecutive observations was used to measure the association between antimicrobial usage and VRE-BSI incidence at the hospital- and care-unit levels.
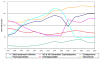
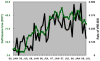
Results: VRE-BSI incidence increased from 0.06 to 0.17 infections per 1,000 patient-days. Hospital VRE-BSI incidence was associated with prior-month ceftriaxone DOTs (incidence rate ratio, 1.38 per 10 DOTs; P = .005). After controlling for ceftriaxone, prior-month cephalosporin usage (class) was not predictive of VRE-BSI (P = .70). Similarly, prior-month usage of piperacillin-tazobactam, ceftazidime, cefepime, cefazolin, or vancomycin was not predictive of VRE-BSI when considered individually (P≥ .4 for all comparisons). The final model suggests that type of intensive care unit was related to VRE-BSI incidence.
Conclusions: Ceftriaxone usage in the prior month, but not cephalosporin (class) or vancomycin usage, was related to VRE-BSI incidence. These findings suggest that an antimicrobial stewardship program that limits ceftriaxone may reduce nosocomial VRE-BSI incidence.
Conflict of interest statement
None of the authors have any conflicts of interest to report in conjunction with this investigation.
Figures
References
-
- Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. - PubMed
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. - PubMed
-
- Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis. 2007;58(2):163–170. - PubMed
-
- System NNIS. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. - PubMed
-
- Scott RD. Division of Healthcare Quality Promotion National Center for Preparedness D, and Control of Infectious Diseases Coordinating Center for Infectious Diseases Centers for Disease Control and Prevention, editor. The Direct Medical Costs of Healthcare-Associated Infections in US Hospitals and the Benefits of Prevention. Vol http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf2009.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
