JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia
- PMID: 22669242
- PMCID: PMC3423125
- DOI: 10.1152/ajpendo.00039.2012
JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia
Abstract
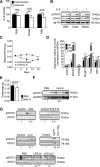
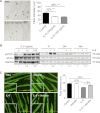
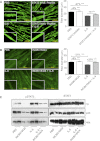
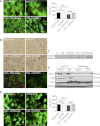
Cachexia, the metabolic dysregulation leading to sustained loss of muscle and adipose tissue, is a devastating complication of cancer and other chronic diseases. Interleukin-6 and related cytokines are associated with muscle wasting in clinical and experimental cachexia, although the mechanisms by which they might induce muscle wasting are unknown. One pathway activated strongly by IL-6 family ligands is the JAK/STAT3 pathway, the function of which has not been evaluated in regulation of skeletal muscle mass. Recently, we showed that skeletal muscle STAT3 phosphorylation, nuclear localization, and target gene expression are activated in C26 cancer cachexia, a model with high IL-6 family ligands. Here, we report that STAT3 activation is a common feature of muscle wasting, activated in muscle by IL-6 in vivo and in vitro and by different types of cancer and sterile sepsis. Moreover, STAT3 activation proved both necessary and sufficient for muscle wasting. In C(2)C(12) myotubes and in mouse muscle, mutant constitutively activated STAT3-induced muscle fiber atrophy and exacerbated wasting in cachexia. Conversely, inhibiting STAT3 pharmacologically with JAK or STAT3 inhibitors or genetically with dominant negative STAT3 and short hairpin STAT3 reduced muscle atrophy downstream of IL-6 or cancer. These results indicate that STAT3 is a primary mediator of muscle wasting in cancer cachexia and other conditions of high IL-6 family signaling. Thus STAT3 could represent a novel therapeutic target for the preservation of skeletal muscle in cachexia.
Figures



References
-
- Alvarez B, Quinn LS, Busquets S, Quiles MT, Lopez-Soriano FJ, Argiles JM. Tumor necrosis factor-alpha exerts interleukin-6-dependent and -independent effects on cultured skeletal muscle cells. Biochim Biophys Acta 1542: 66–72, 2002 - PubMed
-
- Argiles JM, Busquets S, Lopez-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care 6: 401–406, 2003 - PubMed
-
- Argilés JM, Busquets S, Toledo M, López-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 3: 263–268, 2009 - PubMed
-
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

