Common variants of the genes encoding erythropoietin and its receptor modulate cognitive performance in schizophrenia
- PMID: 22669473
- PMCID: PMC3459483
- DOI: 10.2119/molmed.2012.00190
Common variants of the genes encoding erythropoietin and its receptor modulate cognitive performance in schizophrenia
Abstract
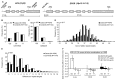
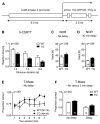
Erythropoietin (EPO) improves cognitive performance in clinical studies and rodent experiments. We hypothesized that an intrinsic role of EPO for cognition exists, with particular relevance in situations of cognitive decline, which is reflected by associations of EPO and EPO receptor (EPOR) genotypes with cognitive functions. To prove this hypothesis, schizophrenic patients (N > 1000) were genotyped for 5' upstream-located gene variants, EPO SNP rs1617640 (T/G) and EPORSTR(GA)(n). Associations of these variants were obtained for cognitive processing speed, fine motor skills and short-term memory readouts, with one particular combination of genotypes superior to all others (p < 0.0001). In an independent healthy control sample (N > 800), these associations were confirmed. A matching preclinical study with mice demonstrated cognitive processing speed and memory enhanced upon transgenic expression of constitutively active EPOR in pyramidal neurons of cortex and hippocampus. We thus predicted that the human genotypes associated with better cognition would reflect gain-of-function effects. Indeed, reporter gene assays and quantitative transcriptional analysis of peripheral blood mononuclear cells showed genotype-dependent EPO/EPOR expression differences. Together, these findings reveal a role of endogenous EPO/EPOR for cognition, at least in schizophrenic patients.
Figures

References
-
- Nissenson AR. Recombinant human erythropoietin: Impact on brain and cognitive function, exercise tolerance, sexual potency, and quality of life. Semin Nephrol. 1989;9:25–31. - PubMed
-
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94. - PubMed
-
- Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24:573–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

